WEBINAR: Validation of a Glycosylation-Independent Antibody Against the Cancer Stem Cell Marker CD133
ABSTRACT
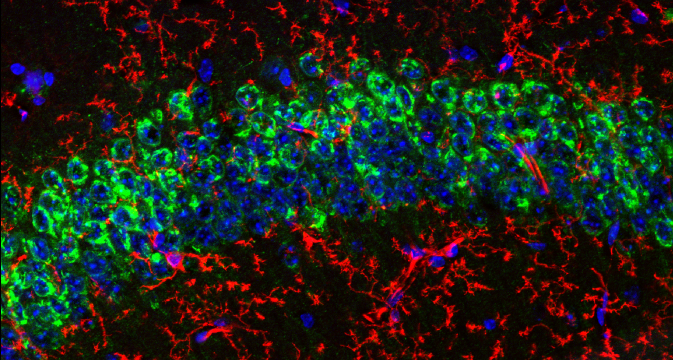
The cancer stem cell hypothesis postulates that a population of self-renewing tumor-initiating cells, termed Cancer Stem Cells (CSCs), may be responsible for driving tumor heterogeneity, metastasis, therapeutic resistance and/or tumor relapse. Tools to identify and characterize putative CSCs are therefore of significant value for the cancer research community. CD133 is a 5- transmembrane (5-TM) cell surface glycoprotein that shows elevated expression in putative CSCs from multiple tumor types. Numerous studies have used antibodies directed against CD133 to isolate putative CSCs for characterization, in vitro culture, transplantation and drug discovery studies. However, the most commonly used antibodies used to study CD133+ CDCs are raised against glycosylated CD133 epitopes; this is problematic because the glycosylation status of CD133 varies in response to environmental conditions (e.g., hypoxia) or cell differentiation status.
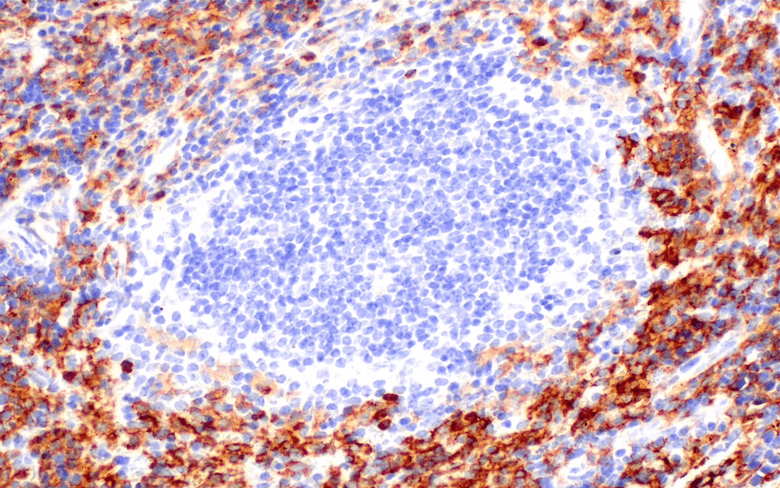
Furthermore, available anti-CD133 antibodies have not been rigorously validated for immunohistochemistry, which is critical for understanding CSC biology in situ. To address this problem, we have developed a recombinant rabbit monoclonal antibody that targets an extracellular, glycosylation-independent epitope of CD133. This reagent [CD133 (D4W4N) XP® Rabbit mAb] has been rigorously validated in Western immunoblot and immunohistochemistry, where it demonstrates robust and specific staining of CD133 protein across diverse cell and tissue types. It is hoped that this reagent will enable accelerated progress in elucidating the role of putative cancer stem cells in the development, metastasis, therapeutic resistance and relapse of tumors.

METHODS
Monoclonal Antibody Generation
CD133 (D4W4N) XP® Rabbit mAb #86781 is a recombinant rabbit monoclonal antibody, generated at Cell Signaling Technology, Inc. using patented XMT® Technology.
Western Blot
Western blot analyses were performed using extracts from cell or tissue extracts, as described in the figure legends. Western blot protocol details can be found at http://www.cellsignal.com/wbprotocol
Immunohistochemistry (IHC)
Paraffin-embedded tissue sections were deparaffinized and rehydrated, then subjected to antigen retrieval in sodium citrate, pH 6.0. Primary antibodies were incubated overnight at 4°C. Detection was performed using SignalStain® Boost IHC Detection Reagent (HRP, Rabbit) #8114 and SignalStain® DAB Substrate Kit #8059.
SUMMARY
CD133 (D4W4N) XP® Rabbit mAb:
A recombinant rabbit monoclonal antibody that detects human CD133 protein in western blot and IHC with exceptional specificity and sensitivity.
Detection of CD133 is independent of protein glycosylation status (determined by western blot).
May be used to detect or visualize CD133 in human cell lysates or formalin-fixed paraffin-embedded (FFPE) human normal and tumor tissues.
Exhibits greater sensitivity in IHC when compared to available anti-CD133 antibodies.