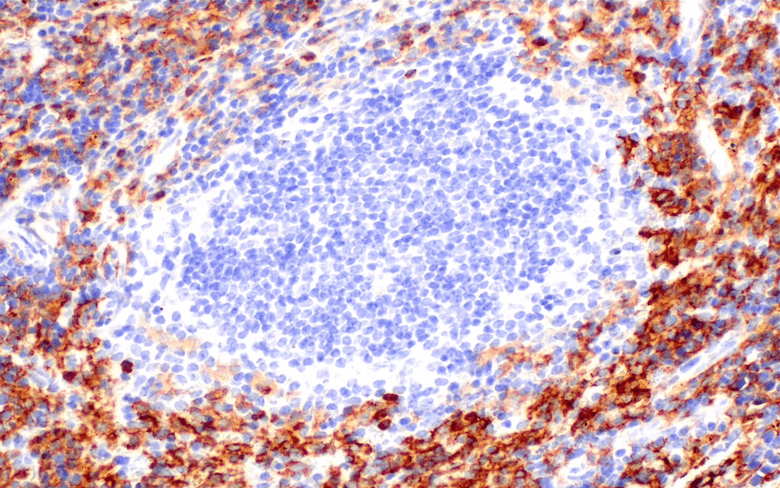
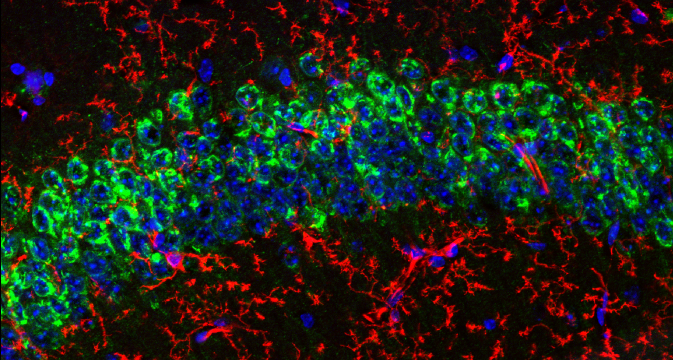
The clinical validation of immune checkpoint inhibitors as immunotherapeutic agents for a variety of cancers has revolutionized the field of cancer therapy. While significant improvement in patient outcome has been observed with previously untreatable tumors, not all patients respond to these drugs (1). An advanced understanding of the immune regulatory context of the tumor microenvironment (TME) is required to harness the power of the antitumor immune response. This will allow identification of novel therapeutic targets and potential biomarkers that can predict response to therapy (2, 3).
Our mIHC application notes and poster resources explore the protocol and technical considerations for selecting and using antibodies in mIHC to assess immunosuppression mediated by myeloid cells in FFPE tissue samples.
.png?width=604&name=18_IHC_010_TME_Appnote_image_1600X800%20(2).png)
Download our mIHC application notes and posters
References
1. Sharma, P. and Allison, J.P. (2015) Cell 161, 205–214.
2. Mahoney, K.M. and Atkins, M.B. (2014) Oncology (Williston Park) 28 (suppl 3), 39–48.
3. Elliott, L. A. et al. (2017) Front Immunol.