CST® anti-CAR linker antibodies won The Innovation Award from CiteAb in 2023. Read on to learn why!
—
Chimeric antigen receptor (CAR) T-cell therapy is a revolutionary and rapidly evolving type of adoptive cell immunotherapy that has shown tremendous promise in leveraging the body’s own immune system to direct an antitumor response. As new CAR-T therapies are developed, detection reagents for characterizing surface-expressed CARs will be critical for the validation and optimization of new therapeutic options.
However, as new CARs are designed against novel tumor antigens, unique detection reagents must be developed and verified for every iteration to ensure CAR surface expression. Not only is this process costly and time-consuming, but issues with non-specific detection can cause further delays.
But what if there were a versatile detection reagent that could identify and confirm the presence of your CAR, regardless of its antigen specificity? CST has developed a panel of detection reagents that are designed to recognize a broad range of CARs on engineered immune cellls: Anti-CAR linker antibodies.
These first-to-market reagents can be incorporated into multiparametric flow cytometry panels for monitoring CAR expression, trafficking, and persistence in preclinical models.
<< Explore the CAR Linker products, including available conjugates >>
Current Approaches: Detecting CAR Surface Expression Using Flow Cytometry
To understand how this novel CAR detection technology works, let’s first explore CAR-T cell structure and function.
CARs are engineered receptors that are designed to modulate T-cell response and redirect it toward a tumor antigen of self-origin. As the name suggests, the receptor is a chimeric protein that can be broken into four main components: an extracellular target antigen-binding domain (e.g., a single-chain variable fragment, or scFv), a spacer or hinge domain, a transmembrane domain, and at least one intracellular signaling domain (Figure 1). The successful combination of these elements redirects the CAR-T cell to identify the selected antigen and kill the tumor cell.1
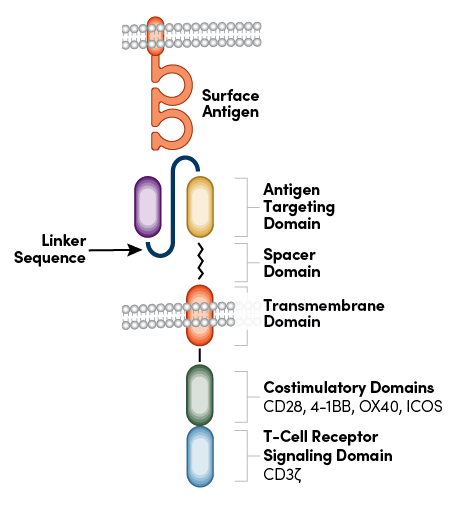 Figure 1: CAR structure detailing the antigen targeting domain and linker sequence.
Figure 1: CAR structure detailing the antigen targeting domain and linker sequence.
When engineering CAR-T cells during discovery and preclinical research, scientists need to verify the cells are functional, in part, by monitoring CAR surface expression using flow cytometry.
Most current detection methods rely on reagents that bind to the extracellular domain of the CAR and leverage specific sequences within either the variable heavy or variable light domains of the scFv or hinge regions.
Depending on the class of CAR-T detection reagent used, available solutions often lack specificity, may not easily be incorporated into flow cytometry panels, or only have utility for detecting only a single CAR.
What are anti-CAR linker antibodies and how do they work?
Instead of targeting sequences within the variable heavy and variable light domains or the hinge region, the new CST anti-CAR linker antibodies target the linker sequence between the variable heavy and variable light domains of the scFv. Because most scFv-based CARs contain either a repeating glycine-serine linker sequence (G4S Linker) or Whitlow Linker sequence,2 anti-G4S antibodies and anti-Whitlow antibodies can be used to monitor the surface expression of virtually any scFv-based CAR using flow cytometry (Figure 2).
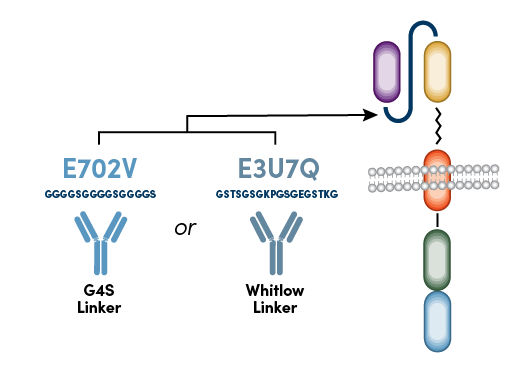
Figure 2. CST anti-CAR linker antibodies recognize either the G4S linker or Whitlow linker sequences.
This is true for any CAR containing these linker sequences, including those that target CD19 and CD20 antigens, which are cell surface molecules that are highly expressed in B cell malignancies.
 Flow cytometric analysis of live pan-CD3+ T cells isolated from human PBMCs and engineered to express an scFv-based anti-CD20 CAR containing the G4S linker using G4S Linker (E7O2V) Rabbit mAb (PE Conjugate) #38907 (right) or concentration matched Rabbit (DA1E) mAB IgG XP Isotype Control PE Conjugate #5742 (left). Tag Blue fluorescent protein (TagBFP) is co-expressed with the CAR. Data courtesy of Micahel Kvrjak, Lohmueller lab (University of Pittsburgh).
Flow cytometric analysis of live pan-CD3+ T cells isolated from human PBMCs and engineered to express an scFv-based anti-CD20 CAR containing the G4S linker using G4S Linker (E7O2V) Rabbit mAb (PE Conjugate) #38907 (right) or concentration matched Rabbit (DA1E) mAB IgG XP Isotype Control PE Conjugate #5742 (left). Tag Blue fluorescent protein (TagBFP) is co-expressed with the CAR. Data courtesy of Micahel Kvrjak, Lohmueller lab (University of Pittsburgh).
As with all CST products, these antibodies have been rigorously validated using our application-specific approach to antibody validation and following the Hallmarks of Antibody Validation methods. This includes testing on relevant model systems, including primary human T cells expressing CARs directed against distinct tumor antigens. The antibodies are available in three a variety of conjugates and can also be custom-conjugated using our custom conjugation services.
 The result is a versatile, validated CAR detection reagent that can help speed your discovery research or drug development process by removing unnecessary and time-consuming steps.
The result is a versatile, validated CAR detection reagent that can help speed your discovery research or drug development process by removing unnecessary and time-consuming steps.
The table below shows some of the anti-CAR linker antibody conjugates and formulations, but more products are always being added. For a continually updated list, refer to our product catalog, which now includes CAR linker-matched antibody pairs, cell enrichment kits, and more. Additionally, you can find fluorochrome-conjugated antibodies for CAR-T cell phenotyping and multiple IHC-validated monoclonal antibodies to analyze CAR targets in tissues.
Rapid Discovery and Development of CAR-T Therapies
Using the body’s own immune system as an anticancer therapy has long been a dream of cancer researchers–a dream that has been made a reality with CAR-T therapies. Referred to as a “living drug,” treatment using CAR-T therapy has been shown to be effective for decades after a single infusion.
"[This] innovative new product [CST anti-CAR linker antibodies] has the potential to have far-reaching impact on cancer patients, helping in the development and optimisation of new treatments." ~ CiteAb 2023 Innovation Awards
The success of these revolutionary therapies for treating hematologic malignancies has paved the way for the development of novel therapies, most notably solid tumors and diseases of senescence, such as fibrotic liver disease and diabetes.3-5 As this promising treatment continues to be at the forefront of immunotherapy, new therapies currently in development will undoubtedly be game-changers for patients with limited options and hard-to-treat cancers.
Learn More:
- Blog: Faster Immuno-Oncology Research with Rapid T Cell Activation & Expansion
- Learn more about our CAR-T therapy research tools.
- Explore the CAR Signaling Networks Interactive Pathway.
- Watch the CAR-T video series for more information about how CAR-T cells are engineered and to explore current research challenges.
Select References:
- Labanieh L, Majzner RG, Mackall CL. Programming CAR-T cells to kill cancer. Nat Biomed Eng. 2018;2(6):377-391. doi:10.1038/s41551-018-0235-9
- Whitlow M, Bell BA, Feng SL, et al. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993;6(8):989-995. doi:10.1093/protein/6.8.989
- Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, Ganguly N. CAR T cell therapy: A new era for cancer treatment (Review). Oncol Rep. 2019;42(6):2183-2195. doi:10.3892/or.2019.7335
- Li JH, Chen YY. A Fresh Approach to Targeting Aging Cells: CAR-T Cells Enhance Senolytic Specificity. Cell. 2020;27(2):192-194. doi:10.1016/j.stem.2020.07.010
- Tenspolde, M., Zimmermann, K., Weber, L. C., Hapke, M., Lieber, M., Dywicki, J., Frenzel, A., Hust, M., Galla, M., Buitrago-Molina, L. E., Manns, M. P., Jaeckel, E., & Hardtke-Wolenski, M. (2019). Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. Journal of Autoimmun. 2019;103:102289. doi:10.1016/j.jaut.2019.05.017
CST and Cell Signaling Technology are trademarks or registered trademarks of Cell Signaling Technology, Inc. Alexa Fluor is a registered trademark of Life Technologies Corporation. All other trademarks are the property of their respective owners. Visit cellsignal.com/trademarks for more information. 22-BPA-10837