Astrocytes play an important role in the central nervous system (CNS), supplying nutrients to neurons, protecting against oxidative stress, and regulating inflammation after injury or infection. Following CNS trauma, astrocytes undergo an activation process known as astrogliosis―a reactive, hypertrophic growth and expansion of the cells that protects uninjured tissue by controlling inflammation, neutralizing harmful byproducts, and preventing the spread of cellular stress. While astrogliosis helps isolate brain damage and aids in recovery, in severe cases, it can exacerbate inflammatory damage and form glial scars that inhibit future neural repair and regeneration.1
Astrocyte activation is not just related to injury; it is also a hallmark of neurodegenerative diseases such as epilepsy, multiple sclerosis, and Alzheimer's. Understanding its pathophysiology is an important area of neuroscience research that could aid in the development of improved approaches for treating traumatic brain injury (TBI), spinal injury, stroke, and neurodegenerative disease.
|
|
Browse the Astrocyte Markers Antibody Sampler Kit #50748, which includes reagents for some of the targets discussed in this post. |
|
Multiplex immunofluorescence (IF) is a common method for investigating astrocyte biology. By leveraging multiple cell type markers, researchers can examine astrocytes in the context of their interactions with neurons, microglia, and other glial populations, while also uncovering insights about subcellular localization and astrocyte morphology within affected brain regions.
In addition to IF, Western blotting can be a powerful complementary method for studying astrocyte activation. Many researchers begin by using WB to screen and quantify proteins across different samples and experimental conditions, then select a subset of markers for deeper interrogation using multiplex IF.
Choosing antibodies that have been validated for use in both WB and IF—and that have been proven to work in relevant model systems and tissue types—can help streamline project execution. This helps ensure reliable, accurate detection across techniques, enabling reproducible results.
This blog explores some of the key astrocyte and glial cell markers, along with highly validated CST recombinant monoclonal antibodies for reliably detecting astrogliosis.
Glial Cell & Reactive Glial Cell Markers
Glial cell density, size, and distribution are powerful indicators of CNS health. These are some of the most commonly used markers when studying astrogliosis:
|
|
#1. GFAPGlial Fibrillary Acid Protein (GFAP) is the most highly studied protein among glial cells. It provides structural stability to glial cells and is upregulated by fibroblast growth factors following injury. GFAP is the primary marker for astrogliosis and scar formation.1 |
|
|
#2. ALDH1L1Aldehyde Dehydrogenase 1 Family Member L1 (ALDH1L1) is a glial cell-specific enzyme involved in folate metabolism and nucleotide synthesis. Unlike GFAP, its expression remains stable following CNS injury, making it a useful marker for astrocytes, regardless of cellular status.2 |
|
|
#3. VimentinVimentin is an intermediate filament protein expressed in activated astrocytes and neural progenitor cells. It is commonly used along with GFAP to identify early astrogliosis at the onset of cytoskeletal changes following injury.3 Co-labeling with other markers, like F4/80, which is observed in microglia and subset populations of dendritic cells, can provide valuable information. |
Transport & Signaling Protein Markers
Glial cells regulate glutamate levels and use gap junctions for rapid, synchronized signaling. The following proteins play prominent roles in astrogliosis:

|
#4. EAAT1/EAAT2Excitatory Amino Acid Transporters (EAAT1/EAAT2) protect neurons from glutamate-induced excitotoxicity. They are frequently upregulated during astrogliosis, and can provide additional insight into reactive glial cells and are being explored as potential therapeutic targets.4 |
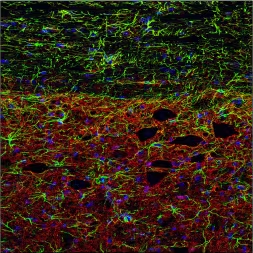
|
#5. Cx43Connexin 43 (Cx43) is a 43 kDa protein that forms gap junction channels, connecting vast networks of glial cells through diffusion of small molecules such as calcium. Cx43 is upregulated by astrocytes in response to CNS injury, potentially as a protective mechanism to counteract the effects of calcium surges from dying cells.
|
Inflammatory Status Markers
Astrocytes can be protective or damaging depending on the circumstances. These markers help identify pathophysiological phenotypes and determine whether reactive astrogliosis is associated with inflammation or repair:
|
|
#6. S100bA calcium-binding protein, S100b is involved in cell cycle regulation and is expressed by a subset of blood vessel ensheathing astrocytes. It binds with the receptor for advanced glycation end products (RAGE) through autocrine, paracrine, and endocrine signaling. As a damage-associated molecular pattern (DAMP) protein, S100b plays a role in neuroinflammation following brain injury. Its upregulation coincides with astrogliosis, and because it is detectable in blood, urine, and serum, S100b is an attractive biomarker for astrogliosis and neurodegenerative diseases.5 |
|
|
#7. Toll-Like ReceptorsToll-like receptors (TLRs) are a family of immune response protein receptors involved in pattern recognition (PAMP) and DAMP signaling that can be used to determine the state of astrogliosis. The TCR subtype activated dictates astrocytic phenotype: TLR2 and TLR4 activation are inflammatory, and TLR3 is anti-inflammatory. View the Toll-like Receptor (TLR) Signaling Pathway to explore relevant CST products. |
|
TNF-α (D2D4) XP Rabbit mAb #11948
|
#8. TNF-αTumor necrosis factor α (TNF-α) is a proinflammatory cytokine upregulated by astrocytes after a CNS injury. It is an early indicator of astrocyte activation, with its expression preceding glial scar formation.6 |
|
|
#9. TGF-βTransforming growth factor β (TGF-β) is a cytokine that helps regulate neuroinflammation by suppressing proinflammatory signals like TNF-α, IL-1β, and NF-kβ. However, in astrocytes, TGF-β activates GFAP and vimentin expression, leading to astrogliosis and scar formation. Examining the spatial and temporal expression patterns of TGFβ has been used in studies of neurodegeneration and astrogliosis.7 |
|
|
#10. Stat3Signal transducer and activation of transcription 3 (Stat3) is a transcription factor activated by cytokines and growth factors responding to injury, including TGF-β and IL-6. When activated, phospho-Stat3 promotes astrogliosis and scar formation.8 |
Extracellular Matrix Markers
The extracellular matrix (ECM) plays a vital role in astrocyte function, CNS repair, and neuroinflammation. These markers help track ECM remodeling and its impact on astrogliosis:
|
|
#11. MMPs (Matrix Metalloproteinases)Matrix Metalloproteinases (MMPs) are a family of calcium-dependent zinc peptidases involved in wound repair, ECM remodeling, cell migration, and angiogenesis. MMPs also contribute to pathophysiological processes, including neuroinflammation, wound healing, and cancer. |

CD11b/ITGAM (E6E1M) Rabbit mAb #17800
|
#12. IntegrinsIntegrins (for example, αVβ3) are transmembrane proteins that regulate cell migration, adhesion, and signaling in astrocytes. Proinflammatory cytokines increase integrin expression, leading to the mobilization of astrocytes and astrocyte activation. The αVβ3 integrin subtype is particularly relevant to astrogliosis, as its overexpression activates Cx43 and NF-kβ, key players in inflammation and glial scar formation.11 Upstream of astrocyte activation, microglia activation may take place. Examining CD11b/ITGAM expression can provide additional information about the role of microglia in astrogliosis. |
Your Antibody Toolkit for Studying Astrogliosis
Cell Signaling Technology provides high-quality antibodies for these astrogliosis and neuroinflammation markers, along with expert support to help you build a tailored panel for your research.
CST also offers a growing portfolio of chimeric monoclonal antibodies, featuring mouse, horse, and feline host species against important neuronal and glial markers. The parent rabbit monoclonals have been validated for both WB and IF, while the chimeric antibodies can be used in multiplex IF experiments.
Browse the latest CST chimeric monoclonal antibodies for multiplex IF:
25-NDG-34900







.jpg?width=210&height=210&name=3709_TGF-beta%20antibody%20(1).jpg)








