It’s a refrain you’ve likely said before: “Give me anything but rabbit!” While rabbit antibodies are often praised for their high affinity and specificity, using multiple rabbit reagents in a single immunofluorescence (IF) experiment can be challenging. If your research requires visualizing two or more protein targets at the same time, multiplexing with rabbit monoclonals can mean sacrificing sensitivity for flexibility—or worse, having to overhaul your protocol to study all your markers of interest.

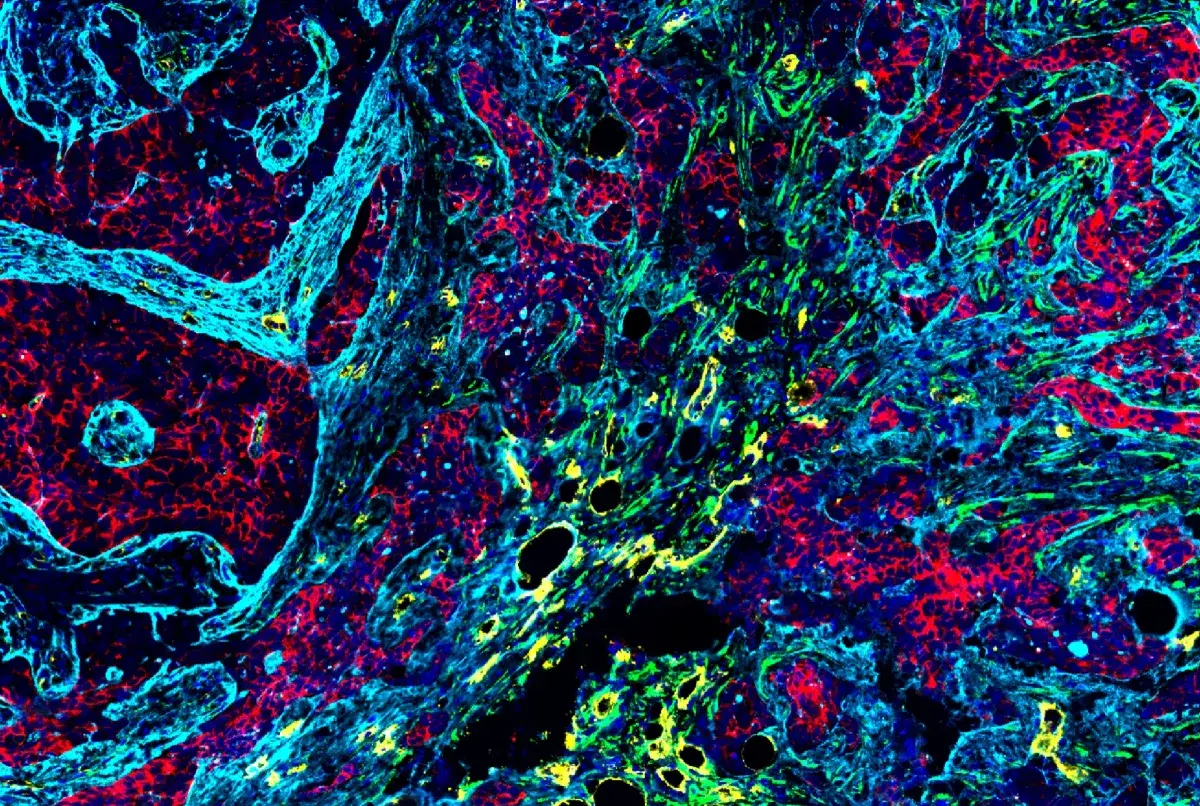
IF analysis of fixed frozen brain from an amyloid mouse model of Alzheimer's disease using chimeric CD68 (feline, green) and TMEM119 (mouse, magenta) antibodies to assess microglial activation state. β-Amyloid (D54D2) XP® Rabbit mAb #8243 (red) was detected using an anti-rabbit Fc-specific secondary antibody. Blue = ProLong Gold Antifade Reagent with DAPI #8961.
But what if you could use the same antibodies you already know and trust, just with a different host species? This is where CST® chimerics come in. Our portfolio of recombinant chimeric antibodies can easily fit into your existing workflow to enable up to four-plex IF without the need for new instrumentation.
What is a monoclonal chimeric antibody?
A recombinant chimeric antibody is an engineered monoclonal antibody that combines a portion of a parent antibody, in this case, rabbit, with a portion of another species’ sequence, like mouse, horse, or feline. Like all recombinant monoclonal antibodies, chimerics are produced using in vitro manufacturing and DNA cloning without the need for animals.
 Put simply, CST chimeric antibodies take the trusted, high-performance clones you already use and re-engineer them with a new host species. The result is a recombinant monoclonal antibody with the same specificity and performance as the original rabbit clone, and that can work together seamlessly in your multiplex IF experiments.
Put simply, CST chimeric antibodies take the trusted, high-performance clones you already use and re-engineer them with a new host species. The result is a recombinant monoclonal antibody with the same specificity and performance as the original rabbit clone, and that can work together seamlessly in your multiplex IF experiments.
This opens up a whole new realm of possibilities that fit within your existing workflow.
Why multiplex with chimeric antibodies?
Multiplexing using primary monoclonal antibodies from different animal hosts is the gold standard in immunofluorescence for neuroscience research. Here’s why:
Easily Integrates Into Your Existing Workflow
You don’t need new equipment, new buffers, or a new protocol. Chimeric antibodies are compatible with your existing set-up and current multiplexing method, and are amenable to high-content analysis. They use the same IF protocol as their parent rabbit clones, so you can easily integrate them into your experiments without a steep learning curve.
Ability to Access More Colors
CST chimeric antibodies are available as horse (IgG4), feline (IgG1), and mouse (IgG2a) host species, allowing you to expand your multiplex panel to include more targets within the same sample. Since they can be detected with species-specific secondary antibodies in four different fluorophore conjugations (see the table below), you can expand your experiments without worrying about cross-reactivity.
 IF analysis using chimeric antibodies for CD11b/ITGAM (mouse, green) and S100B (feline, red), to identify astrocytes and disease-associated microglia surrounding amyloid plaques using fixed frozen brain tissue in an amyloid mouse model of Alzheimer's disease. The rabbit primary antibody, HS1 (D5A9) XP® Rabbit mAb #3892 (gray) was detected using an anti-rabbit Fc-specific secondary antibody. Blue = ProLong Gold Antifade Reagent with DAPI #8961.
IF analysis using chimeric antibodies for CD11b/ITGAM (mouse, green) and S100B (feline, red), to identify astrocytes and disease-associated microglia surrounding amyloid plaques using fixed frozen brain tissue in an amyloid mouse model of Alzheimer's disease. The rabbit primary antibody, HS1 (D5A9) XP® Rabbit mAb #3892 (gray) was detected using an anti-rabbit Fc-specific secondary antibody. Blue = ProLong Gold Antifade Reagent with DAPI #8961.
Visualization of Low-Abundance Targets
Directly conjugated antibodies can be a convenient multiplexing option, but for targets with low expression levels, they may not provide a strong enough signal. Used with a fluorophore-conjugated secondary antibody, chimeric antibodies offer the same high affinity and low background as the original CST clone and outperform direct fluorophore-conjugated antibodies.
Cost Effectiveness
For IF experiments with up to four targets, chimeric antibodies are a simple and cost-effective solution. Unlike complex high-plex systems that require specialized equipment, extensive optimization, and expensive oligo- or barcoded reagents, chimeric antibodies use your existing instrumentation along with standard secondary antibodies. This saves you from the expense of new training, complex reagents, or specialized imaging platforms. Moreover, chimeric antibodies can be purchased in convenient, affordable 20 µL trial sizes. This means you can buy just as much as you need to run preliminary experiments before committing to a particular SKU.
How to Use a Chimeric Antibody in Your Workflow
Using a chimeric antibody is a simple addition to your standard IF protocol.
After fixing and permeabilizing your cells or tissue, you can add your chimeric antibodies, along with a rabbit primary, during the primary antibody incubation step. Then, after washing off the primaries, add the appropriate fluorophore-conjugated, species-specific secondary antibodies. After incubation with your secondary antibodies, the cells or tissue can be mounted using your standard IF protocol.
There’s one important thing to keep in mind, however: Since the chimeric antibody has part of the same antigen-binding region as its rabbit parent clone, you need to use an Fc-specific secondary antibody of a different species to avoid cross-reactivity.
What is an Fc-specific secondary antibody? An Fc-specific secondary is a type of secondary antibody designed to bind only to the Fc region (the "stalk" of the Y-shaped antibody molecule) of a primary antibody. Since the remaining portion of the chimeric antibody retains the same rabbit-host binding site as the original parent clone, it would be recognized by a pan-rabbit secondary antibody, leading to false signals. Using an Fc-specific secondary antibody ensures you’re imaging distinct signals from each host and prevents unexpected overlap.
The table below includes links to the secondary antibodies that are compatible with CST chimerics, which are available in four different excitations.
For example, if you are using a rabbit primary antibody and a feline chimeric antibody, you should use an anti-rabbit Fc secondary antibody to detect the rabbit primary and an anti-feline secondary antibody to detect the chimeric antibody, as shown below. This ensures that each secondary antibody will bind only to its intended target, eliminating the chance for false signal.
IF analysis of fixed frozen mouse cerebellum using PSD95 (D27E11) Horse Chimeric mAb #19622 (gray), MAP2 (D5G1) XP Rabbit mAb #8707 (green), Synaptophysin (D8F6H) Feline Chimeric mAb #76788 (red), and ProLong Gold Antifade Reagent with DAPI #8961 (blue). The rabbit primary antibody MAP2 (D5G1) XP® Rabbit mAb #8707 is detected using Goat Anti-Rabbit IgG, Fc Fragment Antibody (Alexa Fluor® 647 Conjugate) #46023.
Antibodies You Can Trust
Consistent, reliable antibodies are critical for accurate results. That's why every chimeric antibody is validated against its parent clone to ensure it retains the same high performance you expect from CST. When paired with a quality CST secondary antibody, CST chimeric antibodies are guaranteed to provide consistent, reproducible results—the first time and every time.
As of October 2025, available products are validated on fresh frozen tissue using immunofluorescence. Currently, these antibodies can be purchased in a standard, glycerol-based formulation. Carrier-free, BSA-free, and custom concentrations are also available, as are bulk orders.
You don’t need to wrestle with unfamiliar protocols or new instrumentation to achieve in-depth, robust results. Start focusing on the science with familiar, streamlined multiplex experiments using CST chimeric antibodies.
Additional Resources
- Blog: Designing Multiplex IF Experiments for Neuroscience Research with Chimeric Antibodies
- Blog: Strategies for Successful Multiplex IF Experiments Using Conjugated Antibodies
- Blog: Fluorescent Staining Using Multiple Antibodies: Two Common Techniques
- Resource Center: Multiplexing and Spatial Biology
Alexandra Foley, CST Marketing Content Manager, contributed to writing this blog post. 25-NDG-41450