Part 1 of a four-part series on successful immunofluorescence. Read Part 2: Experimental Controls, Part 3: Fixation and Permeabilization, and Part 4: Antibody Dilution and Incubation Conditions.
—
After months of hard work, your research has zeroed in on a hypothesis you can test with immunofluorescence (IF). But now you have to make a choice. How do you decide which antibody to use to get reliable IF results? How do you know if the images are accurately reporting the target's localization? We explore some considerations in this post.
Validate Specificity to Avoid Misleading IF Results
The primary antibody is a critical component of an IF experiment, and its performance directly affects data quality. Detection of a specific band on a western blot (WB) is not sufficient to guarantee a chosen antibody will perform in IF. WB analysis subjects proteins to harsh reducing/denaturing conditions that alter protein structure, so an epitope recognized by an antibody approved for WB may be buried and/or inaccessible for IF, where proteins remain in their native state.
To illustrate why IF-specific validation is critical to ensure accurate results, we tested a CST® antibody validated for IF against another vendor's validated antibody, which was marketed for use in IF applications but only validated in WB.
IF Validation of an α-Synuclein Antibody
The α-Synuclein protein is highly expressed in the brain and its dysfunction plays a role in neurodegenerative diseases, such as Parkinson’s disease. In healthy tissue, α-Synuclein is expected to localize to presynaptic terminals, where it associates with synaptic vesicles.
CST scientists compared α-Synuclein (D37A6) XP® Rabbit mAb #4179 side-by-side with another company’s α-Synuclein antibody, using each at the manufacturer’s recommended dilution. Both antibodies performed as expected in WB (Figure 1, left). In IF (Figure 1, right), punctate staining consistent with presynaptic localization of α-Synuclein was observed in midbrain sections for #4179, while the other company’s antibody showed a less punctate pattern and, critically, it misreported localization in nuclei or soma (Figure 1, bottom right arrows).
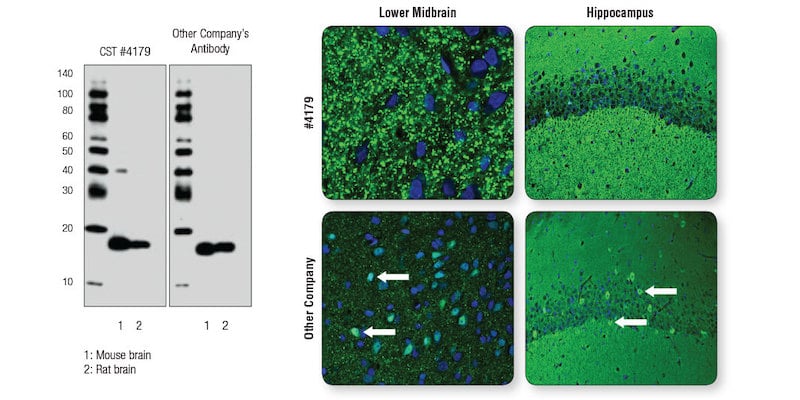
Figure 1. A “clean” WB is not sufficient to ensure performance and reliability of an antibody in IF analysis. Left: WB analysis of extracts from mouse and rat brain using α-Synuclein (D37A6) XP® Rabbit mAb #4179 or the other company’s antibody to α-Synuclein . Right: Confocal IF analysis (of mouse lower midbrain and hippocampus sections using #4179 (top row) or the other company’s antibody (bottom row). Neuronal soma/ nuclei that are mislabeled for α-Synuclein with the other company’s antibody are noted with white arrows.
This experiment illustrates the importance of application-specific validation.
Best Practices for Successful Immunofluorescence Experiments
Read the rest of the Successful Immunofluorescence series, including posts discussing the design of controls for IF, considerations for fixation and permeabilization, and antibody incubation conditions:
- Part 1: Successful Immunofluorescence: The Importance of Validation
- Part 2: Successful Immunofluorescence: Experimental Controls
- Part 3: Successful Immunofluorescence: Fixation and Permeabilization
- Part 4: Successful Immunofluorescence: Antibody Dilution and Incubation Conditions
You can also follow the link below to download the Guide to Successful Immunofluorescence, a resource packed with tips and the 9-step Protocol for a successful immunofluorescence experiment.
Additional Resources:
For more tips for achieving successful immunofluorescence, check out the blogs below:






/42157_chimeric%20antibody%20blog%20featured3.webp)
