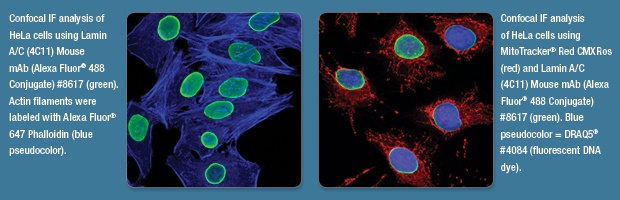
A picture is worth a thousand words, or in the case of immunofluorescent (IF) imaging, a thousand proteins. The images used to illustrate a scientific experiment should convey as much information as the text itself. Here at CST, we pride ourselves in the quality of our antibodies and our rigorous validation process. When we approve our primary antibodies for IF, we like to showcase them using high-quality images generated in-house. Beyond our recommended IF protocols, here are some additional considerations to make when planning your IF staining.
Step 1: Cell Health
The health of the cells that you use will come across in your IF images. That plate of cells forgotten in the incubator over the weekend may be multinucleated, have stress granule formation, contamination, or dead cell debris (see below).
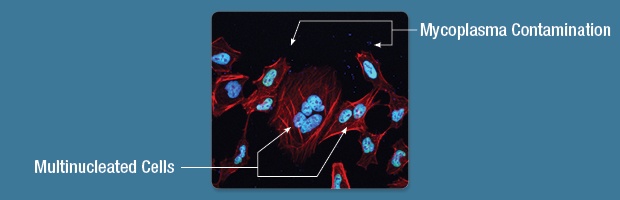
Step 2: Confluence
Sometimes we want to look at the signaling of highly confluent cell cultures, such as for tight junction staining with ZO-2 (e.g., ZO-2 Antibody #2847). However, if cells are over-confluent without any defined edges, you can lose context in your image. On the other hand, if cells are too sparse, your choice of fields to be imaged is limited.
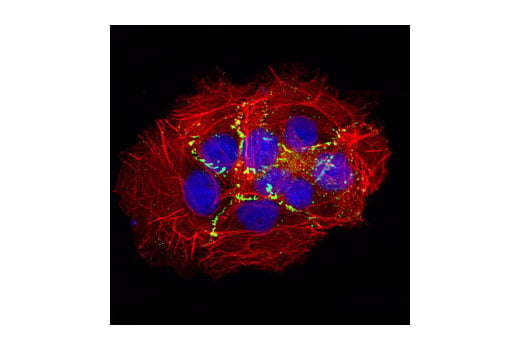
IF analysis of A431 cells using ZO-2 Antibody #2847 (green). Actin filaments have been labeled with DY-554 phalloidin (red). Blue pseudocolor = DRAQ5™ (fluorescent DNA dye).
Step 3: Primary Antibody Selection
The use of a well-validated antibody will make the imaging process smoother. The best results are achieved when all validation work (titration, protocol optimization) is performed prior to final image generation. Grayscale images generated by high-content analysis software are useful for comparing the intensities of antibodies in a plate format (left). Single or two-color images are sufficient for determining optimal working dilutions (center). These early analyses ensure that the final, three-color image is the best representation of the target of interest (right).
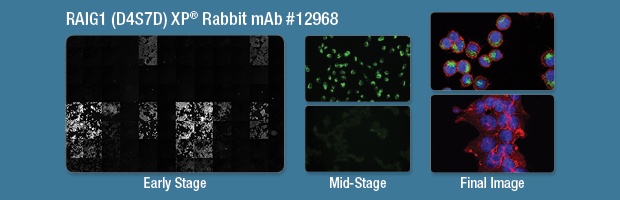
IF analysis of MKN-45 (far, top) and 293T (right, bottom) cells using RAIG1 (D4S7D) XP Rabbit mAb #12968 (green). Actin filaments were labeled with DyLight 554 Phalloidin #13054. Blue pseudocolor = DRAQ5® #4084 (fluorescent DNA dye).
Step 4: Secondary Antibodies and Counterstains
At CST, we typically use Alexa Fluor 488-conjugated secondary antibodies (or an antibody directly conjugated to Alexa Fluor 488) to detect the target of interest, because its green fluorescence is visually striking. Counterstains and DNA dyes should be chosen to help highlight the antibody staining and give context, but not overshadow the staining of interest.
Step 5: Field and Zoom
If possible, choose a field with a moderate number of cells (we aim for more than three) that demonstrates antibody performance—if staining is more heterogenous, be sure to pick a field that demonstrates that. If you’re too zoomed out you may not be able to convey specific subcellular localizations such as mitotics or focal adhesions.
As with any technique, practice makes perfect. The more fluorescent staining experiments you get under your belt, the more comfortable you’ll become and the better your images will be. Even if you have to shoot every field in a well before you get something you like, it will be worth the effort. Good luck and may the fluorophores be with you!