Part two of a four-part series on successful immunofluorescence. Read Part 1: The Importance of Validation, Part 3: Experimental Controls, and Part 4: Antibody Dilution and Incubation Conditions.
—
After months of hard work, your research has honed in on a hypothesis you can test with immunofluorescence (IF). You've chosen antibodies and performed pilot IF experiments (see Part 1: The Importance of Validation), and the localization of the protein appears reasonable.
But how can you be sure the IF data you've acquired represents real biological phenomena? We present two examples of experimental controls in this post.
Experimental Controls for IF
The incorporation of appropriate controls is important to confirm that the only changes between samples are in the experimental variable(s), and that the reagents—including antibodies—are performing as expected. Such controls could involve pharmacological treatments, the addition of extracellular ligands to modulate signaling pathways, or the comparison of cells with differential gene expression (knockout, siRNA, etc.). Typically, variables and controls are performed in parallel such that the fixation step and subsequent processing can be performed at the same time. The type of control used is dependent on the type of experiment.
In the following sections, we provide examples of controls our scientists use to evaluate antibody performance in immunofluorescence experiments, some of which you may want to incorporate to confirm specificity in your cell type.
Knockout Cell Lines to Verify Target Specificity
If you are investigating a target with multiple isoforms, it may be useful to perform controls using knockout cell lines. For example, two genes encode glycogen synthase kinase (GSK-3), designated GSK-3α and GSK-3ß, which are regulated at different serine residues. When performing an IF experiment, you would want to know if the antibody you’re using recognizes one isoform, both, or neither.
This can be confirmed using cells that are known to be positive or negative for the expression of the target of interest. Using wild-type (positive for GSK-3ß), GSK-3α knockout (positive for GSK-3ß), and GSK-3ß knockout (negative for GSK-3ß) mouse embryonic fibroblasts (MEFs) demonstrates that GSK-3ß (D5C5Z) XP Rabbit mAb #12456 specifically recognizes the correct isoform in IF.
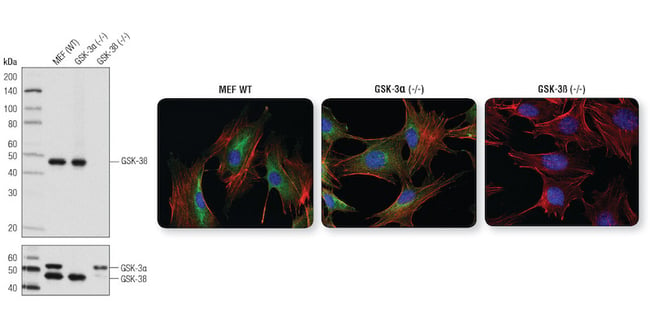
GSK-3ß (D5C5Z) XP Rabbit mAb #12456 is specific for GSK-3ß as demonstrated by WB analysis and IF analysis. Left: WB analysis of extracts from wild-type, GSK-3α (-/-), and GSK-3ß (-/-) MEFs using #12456 (upper) and GSK-3α/ß (D75D3) XP Rabbit mAb #5676 (lower). Right: IF analysis of wild-type MEFs (left), GSK-3α (-/-) MEFs (center) and GSK-3ß (-/-) MEFs (right) using #12456 (green). Actin filaments were labeled with DyLight 554 Phalloidin #13054. Blue pseudocolor = DRAQ5® #4084 (fluorescent DNA dye).
Confirming Phospho-Specificity by Modulating Target Phosphorylation State
Antibodies specific for post-translational modifications (PTMs), including phosphorylation, acetylation, ubiquitination, cleavage, and others, can reveal important information about the biological function of the target protein. In some cases, changes in PTM may coincide with changes in expression and/or localization. For this reason, confirmation of PTM changes in cells can be done using PTM-specific antibodies in conjunction with enzymatic or chemical agonists and/or inhibitors to modulate the activation state of the target protein.
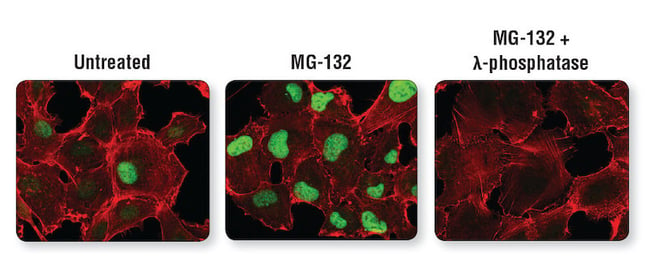
Experimental perturbations confirm PTM-specificity of an antibody. Confocal IF analysis of HT-1080 cells untreated (left) or treated with Protease MG-132 alone (center) or with MG-132 followed by λ-phosphatase (right), using Phospho-Cyclin D1 (Thr286) (D29B3) XP Rabbit mAb #3300 (green). Actin filaments were labeled with DyLight 554 Phalloidin #13054.
Best Practices for Successful Immunofluorescence Experiments
Download the Guide to Successful Immunofluorescence, a resource packed with tips and the 9-step Protocol for a successful immunofluorescence experiment.
Additional Resources:
For more tips for achieving successful immunofluorescence, check out the blogs below:
- 5 Steps to Publication-Worthy IF Images
- 3 Questions to ask before starting your IF experiment
- Fluorescent Staining Using Multiple Antibodies
Read the complete Successful Immunofluorescence Series:
- Part 1: Successful Immunofluorescence: The Importance of Validation
- Part 2: Successful Immunofluorescence: Experimental Controls
- Part 3: Successful Immunofluorescence: Fixation and Permeabilization
- Part 4: Successful Immunofluorescence: Antibody Dilution and Incubation Conditions







