Since 2000, Cell Signaling Technology (CST) has offered a rigorously tested and well-cited polyclonal antibody product that recognizes nitro-tyrosine proteins for western blot applications (WB), Nitro-Tyrosine Antibody #9691. In light of the wider debate on the suitability of polyclonal antibodies for research1,2 to better understand cellular pathways specifically regulated by nitro-tyrosine, CST has developed two recombinant monoclonal antibody products against the same post-translational modification (PTM) for western blot (WB) and proteomics applications:
- WB: Nitro-Tyrosine (D2W9T) Rabbit mAb #92212
- Proteomics: PTMScan® Nitro-Tyrosine Motif [Nitro-Y] Kit #44462
These new reagents, along with the availability of the preexisting polyclonal product, provide users with greater confidence and opportunities to explore nitro-tyrosine in their biological systems.
A Longstanding Target with Modern Relevance
Not to be confused with a Nitrous Oxide System (NOS), popularized to accelerate cars to near-light speed, nitrosylation of tyrosines (NOS) plays a critical role in the lab and in biology. In vitro nitrosylation of tyrosine to yield nitro-tyrosine (also known as 3-nitrotyrosine) has long been utilized as a technique to probe protein structure and interactions.3,4
Ever the innovator, nature has also found ways to modulate nitro-tyrosine. Myeloid-derived suppressor cells (MDSCs) release reactive nitrogen species (RNS) that nitrosylate proteins on cytotoxic T lymphocytes. T-cell activity is attenuated upon nitro-tyrosine formation; and thus, this PTM provides a mechanism for cells to evade immunotherapy.5
Related: Immunology: Which cells have a myeloid lineage and how are they identified?
Separately, the same RNS that generates nitro-tyrosine can be scavenged with uric acid. In animal models for diseases associated with compromised a blood-brain barrier, such as multiple sclerosis, administration of uric acid partially restores blood-brain barrier integrity, presumably by antagonizing nitro-tyrosine formation.6
An introduction to nitro-tyrosine would not be complete without the mention of another tyrosine PTM: Phospho-tyrosine (Figure 1B). In the Src family of protein tyrosine kinases (SFKs), which includes the Lyn tyrosine kinase, the Src homology 2 (SH2) domain negatively autoregulates the enzyme. Formation of either phospho-tyrosine or nitro-tyrosine on a defined site in the SH2 domain prevents inhibition, thereby activating kinase activity.7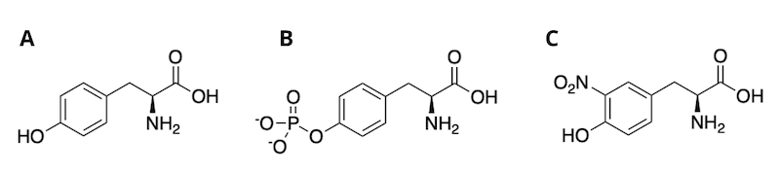 Figure 1. Structures of unmodified tyrosine (A), phospho-tyrosine (B), and nitro-tyrosine (C).
Figure 1. Structures of unmodified tyrosine (A), phospho-tyrosine (B), and nitro-tyrosine (C).
The use of specific reagents to assay for nitro-tyrosine or phospho-tyrosine, which can be achieved using Phospho-Tyrosine (P-Tyr-1000) MultiMab™ Rabbit mAb mix #8954, may clarify the roles of these two naturally occurring PTMs.
A New Monoclonal Western Blot Product
For a pan-PTM antibody with broad reactivity for many substrates containing a specific PTM, polyclonal antibodies are sometimes preferred over monoclonal antibodies due to their ability to detect multiple antigens. However, the antigen design and antibody screening process we use at CST to develop our innovative PTMScan® line of products is able to uncover monoclonal antibodies that exhibit this unique feature—the ability to recognize many substrates containing a specific PTM. This allows researchers to benefit from the advantages of using a monoclonal antibody—namely, antibody binding consistency—while still probing for a single, specific PTM.
For nitro-tyrosine, CST has developed the monoclonal antibody, Nitro-Tyrosine (D2W9T) Rabbit mAb #92212, that recognizes many modified proteins from cells treated with the RNS reagent peroxynitrite in WB applications. PTM specificity was assessed in two ways:
- First, using preincubation of the primary antibody with nitro-tyrosine peptide (Figure 2), and
- Second, using chemical reduction of the nitro-tyrosine modification to an antigen that is not recognized by the antibody using sodium dithionite (Figure 3).8
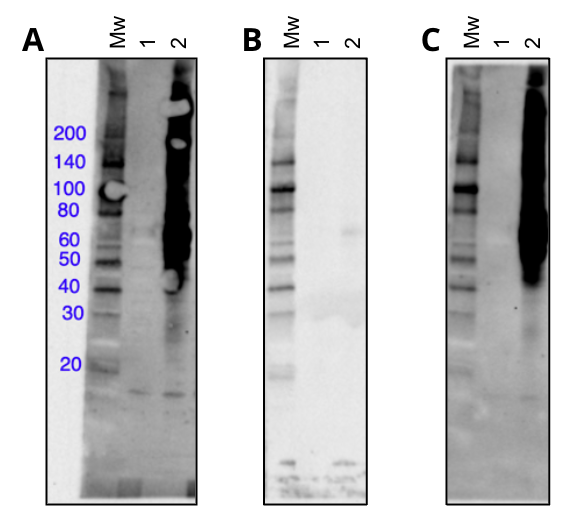
Figure 2. Western blot using the monoclonal antibody Nitro-Tyrosine (D2W9T) Rabbit mAb #92212 in untreated (lane 1) or peroxynitrite treated (lane 2) Hela cells, blocked with nothing (A), a degenerate nitro-tyrosine peptide library (B), or a degenerate phospho-tyrosine peptide library (C).
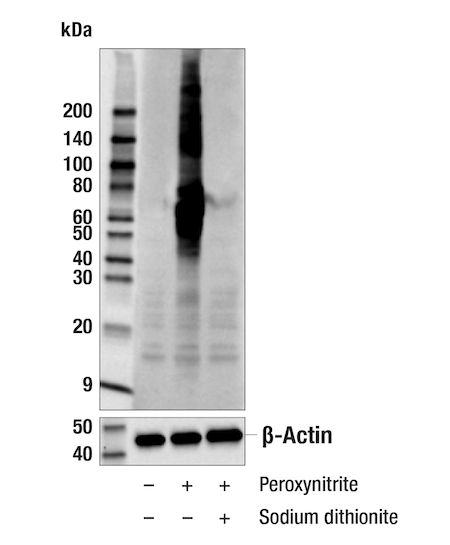
Figure 3. Western blot using the monoclonal antibody Nitro-Tyrosine (D2W9T) Rabbit mAb #92212 in untreated (lane 1), peroxynitrite treated (lane 2) or sodium dithionite treated lane 3) extracts from HeLa cells.
In addition to being specific for nitro-tyrosine, monoclonal antibodies have the added benefit of having a defined sequence, facilitating traceability during manufacturing.
Analyzing Nitro-Tyrosine Using Proteomics: A NEW PTMScan Product
Although WB reagents provide a convenient method to assess nitro-tyrosine levels, it’s difficult to unambiguously identify nitro-tyrosine proteins or discriminate between individual modified sites on those proteins. To identify the proteins and specific nitro-tyrosine sites, enrichment for nitro-tyrosine is crucial. The concept of using a nitro-tyrosine-specific antibody to enrich for PTM-containing peptides was first demonstrated in the 1970s, when a column with immobilized polyclonal antibodies was used to enrich tryptic peptides from an in vitro nitrosylated protein.9
A newer iteration of this concept is the PTMScan technology, a workflow developed by CST that uses PTM-specific antibodies to enrich for modified peptides in a bead slurry rather than column format for ease of use. PTMScan enriched peptides are assayed with liquid chromatography-mass spectrometry (LCMS).
Related: A Revolution in Proteomics Analysis: PTMScan 20 Years Later
CST has released a PTMScan antibody-bead product for the enrichment of nitro-tyrosine peptides, PTMScan® Nitro-Tyrosine Motif [Nitro-Y] Kit #44462. Although PTMScan assays require more sample material compared to WB assays (milligrams compared to micrograms), the ability to unambiguously identify and quantify nitro-tyrosine-modified sites can be an invaluable asset. Additionally, the remaining peptide sample left after nitro-tyrosine enrichment (the flow-through) can be used as input for subsequent PTMScan assays, to monitor other PTMs of interest in a given study. A collaborative research project between CST and Duke University School of Medicine demonstrates an example of such a sequential PTMScan experiment.
Starting with 10 mg of input peptide material from HCT116 cells, one can identify several hundred unique nitro-tyrosine sites with the PTMScan Nitro-Tyrosine Immunoaffinity Beads (Figure 4). The sequence motif analysis of the identified sites suggests that the antibody has little bias for the flanking amino acids. Thus, PTMScan Nitro-Tyrosine Immunoaffinity Beads are a suitable reagent for assaying nitro-tyrosine sites from various species (i.e., mouse, human).
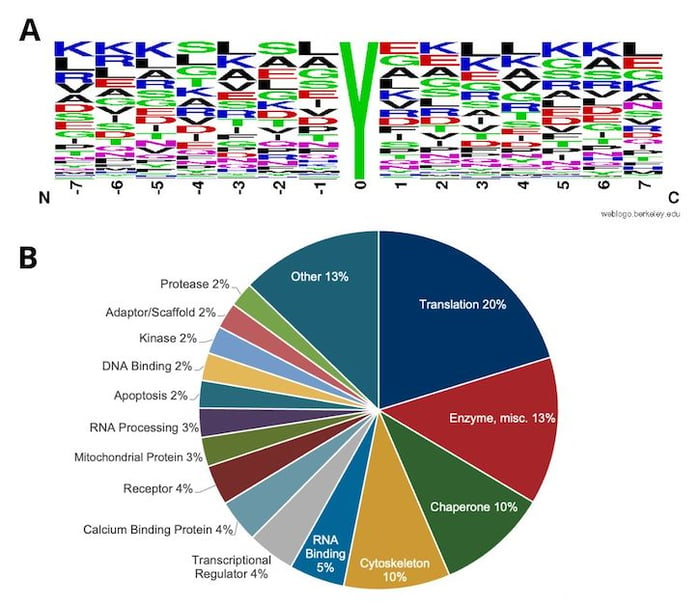 Figure 4. Motif analysis using all nitro-tyrosine peptides enriched and identified by PTMScan Nitro-Tyrosine Motif [Nitro-Y] Kit #44462, spanning over 700 unique sites, from HCT 116 human colon cancer cells (A), with relative category distribution of proteins with Nitro-Tyrosine sites show (B).
Figure 4. Motif analysis using all nitro-tyrosine peptides enriched and identified by PTMScan Nitro-Tyrosine Motif [Nitro-Y] Kit #44462, spanning over 700 unique sites, from HCT 116 human colon cancer cells (A), with relative category distribution of proteins with Nitro-Tyrosine sites show (B).
Old Target, New Tools
In cancer, neurodegeneration, and likely other diseases involving oxidative stress, researchers continue to characterize pathways affected by nitro-tyrosine. With new monoclonal reagents validated for WB and proteomic experiments, users can employ a more comprehensive strategy to form and test hypotheses on this PTM function.
Relevant CST Products
Antibody Products |
Applications |
| Nitro-Tyrosine Antibody #9691 |
WB |
| Nitro-Tyrosine Antibody (D2W9T) Rabbit mAb #92212 |
WB |
| Phospho-Tyrosine (P-Tyr-1000) MultiMab™ Rabbit mAb mix #8954 |
WB, IP, IF, F |
| PTMScan® Nitro-Tyrosine Motif [Nitro-Y] Kit #44462 |
Proteomics |
Select References
- Ascoli CA, Aggeler B. Overlooked benefits of using polyclonal antibodies. Biotechniques. 2018;65(3):127-136. doi:10.2144/btn-2018-0065
- Bradbury A, Plückthun A. Reproducibility: Standardize antibodies used in research. Nature. 2015;518(7537):27-29. doi:10.1038/518027a
- Furth AJ, Hope DB. Studies on the chemical modification of the tyrosine residue in bovine neurophysin-II. Biochem J. 1970;116(4):545-553. doi:10.1042/bj1160545
- Irie M, Suito F. Studies on the state of tyrosyl residues in a ribonuclease from seminal vesicles. J Biochem. 1975;77(5):1075-1084. doi:10.1093/oxfordjournals.jbchem.a130808
- Feng S, Cheng X, Zhang L, et al. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc Natl Acad Sci U S A. 2018;115(40):10094-10099. doi:10.1073/pnas.1800695115
- Kean RB, Spitsin SV, Mikheeva T, Scott GS, Hooper DC. The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into the central nervous system in experimental allergic encephalomyelitis through maintenance of blood-central nervous system barrier integrity. J Immunol. 2000;165(11):6511-6518. doi:10.4049/jimmunol.165.11.6511
- Mallozzi C, Di Stasi AM, Minetti M. Nitrotyrosine mimics phosphotyrosine binding to the SH2 domain of the src family tyrosine kinase lyn. FEBS Lett. 2001;503(2-3):189-195. doi:10.1016/s0014-5793(01)02726-0
- Sangsuwan R, Obermeyer AC, Tachachartvanich P, Palaniappan KK, Francis MB. Direct detection of nitrotyrosine-containing proteins using an aniline-based oxidative coupling strategy. Chem Commun (Camb). 2016;52(65):10036-10039. doi:10.1039/c6cc04575h
- Helman M, Givol D. Isolation of nitrotyrosine-containing peptides by using an insoluble-antibody column. Biochem J. 1971;125(4):971-974. doi:10.1042/bj1250971






