Under the category of necrotic cell death, there are many well-characterized, as well as some newly described, processes of cellular destruction. Contrary to classical thinking, necrotic cell death is not always physical and accidental in nature. Instead, cells may trigger specific programmed self-destruct pathways when they are under stress and other methods of cell death are not feasible.
Antibodies targeting key proteins involved in the canonical and non-canonical pyroptosis pathways are essential tools for researchers studying this unique form of cell death.
Mechanisms and Pathways of Pyroptosis Cell Death
Pyroptosis is a type of programmed necrotic cell death that is activated upon intracellular infections from bacteria, viruses, fungi, and protozoa in the presence of pathogen-associated molecular patterns (PAMPs) or cell-derived damage-associated molecular patterns (DAMPs). It is typically induced in cells of the innate immune system, such as monocytes, macrophages, and dendritic cells.
Pyroptosis is often the primary mode of cell death that may be triggered upon pathogenic infection, and it is thought that other types of cell death, like necroptosis, occur as a secondary process when caspase enzymes are not available. Cells that undergo pyroptosis display morphological characteristics such as cell swelling, membrane blebbing, DNA fragmentation, and eventual cell lysis. However, the nucleus often remains intact, which differs from the nuclear destruction that can be observed during apoptosis and necroptosis.
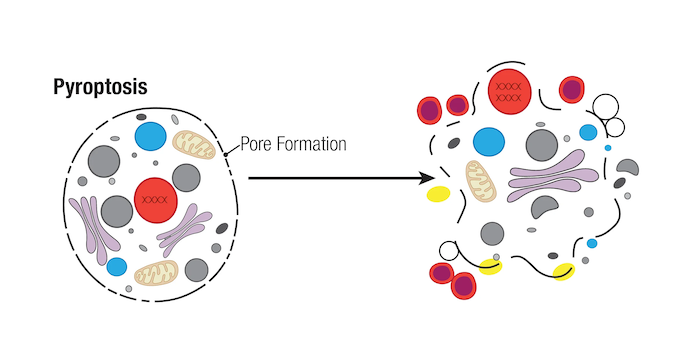
Since DNA fragmentation is random and the nucleus stays intact, pyroptotic cells generate a low, positive signal when analyzed with a TUNEL assay. However, other assays are required to differentiate between pyroptosis, other types of necrosis, and apoptosis.
Antibodies specific to markers such as those described below can be used to identify pyroptosis and distinguish between its different pathways.
Antibody Targets for Studying Pyroptosis
Pyroptosis is mainly characterized by the N-terminal cleavage of gasdermin D (GSDMD), which results in its oligomerization to form a lytic pore in the plasma membrane. This cleavage process relies on the activity of inflammatory caspase-1, caspase-4, caspase-5, and/or caspase-11, which are different from the caspases that are active during apoptosis. Therefore, monitoring the cleavage of GSDMD and its related family members, as well as these inflammatory caspases, are key markers for studying pyroptosis. However, it's important to note that different caspases are involved in different pyroptosis pathways, as described below.
The inflammatory caspases play an important role in the innate immune system and enable immune cell response to bacterial infection, as they are involved in processing and secreting pro-inflammatory cytokines, such as pro-interleukin-1β (pro-IL-1β) and pro-interleukin-18 (pro-IL-18).
There are two distinct pyroptosis pathways, the canonical and non-canonical pathways, which can be further distinguished using distinct markers.
Canonical Pyroptosis Pathway
The canonical pathway of pyroptosis is often described as occurring through a two-step process. In the first priming signal step, Nuclear factor-kappa B (NF-kB) is activated to induce the expression of a number of proteins that will become part of a complex called the inflammasome. Inflammasomes typically consist of a cytosolic-Pattern Recognition Receptor, (i.e., PRRs such as NLRP3 or AIM2-like family members), an adaptor protein (ASC/TMS1), and pro-caspase-1. Analyzing the activation of the inflammasome via the detection of NLRP3 and visualization of ASC speckling is another useful technique to monitor pyroptosis.
In the second activation step, caspase-1 is proteolytically activated before cleaving the cytokines, pro-IL-1β and pro-IL-18, creating their pro-inflammatory forms, IL-1β and IL-18, which are secreted from the dying cell. Importantly, caspase-1 also cleaves the N-terminal fragment of the key protein gasdermin D (GSDMD), allowing it to oligomerize and form a pore in the cell membrane. This leads to the secretion of cytokines, influx of water, and eventual cell rupture.
Caspase-1, NLRP3, and ASC speckling are all essential markers for the canonical pyroptosis pathway.
Non-canonical Pyroptosis Pathway
Alternatively, pyroptosis can be induced via the non-canonical pathway by the intracellular detection of Gram-negative bacterial LPS, which activates caspase-4, caspase-5, and/or caspase-11 (mouse caspase-11) to cleave GSDMD. Therefore, activation of these caspases can help identify the non-canonical pyroptosis pathway.
Since GSDMD is cleaved in both the canonical and non-canonical pathways, monitoring the cleavage and translocation of GSDMD is an important part of identifying pyroptosis occurring via either pathway.
Pyroptosis in Disease Research
Investigating the mechanisms of pyroptosis may result in therapeutic benefits for cancer, autoimmune, neurodegenerative diseases, and more. The potential for pyroptosis to mediate cancer is evidenced by research demonstrating that omega-3 fatty acids activate pyroptosis in triple-negative breast cancer cells; however, the mechanism remains to be elucidated. In inflammatory bowel disease, activation of the inflammasome and caspase induction is indicative of pyroptosis, and this may also be a promising direction of research to develop novel therapeutics.
Additional Resources
To learn more about the mechanisms, morphology, and key proteins involved in many types of cell death, download the guide below:
Read the additional blog posts in the Mechanisms of Cell Death series:
- Mechanisms of Cell Death: Apoptosis
- Mechanisms of Cell Death: Necrosis & Necroptosis
- Mechanisms of Cell Death: Ferroptosis
Explore our CST® TUNEL kits, which robustly detect cells undergoing apoptosis and other forms of programmed cell death, as well as our pyroptosis antibody sampler kits:
Select References
- Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486-541. doi:10.1038/s41418-017-0012-4
- Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26(1):99-114. doi:10.1038/s41418-018-0212-61.
- Escobar ML, Echeverría OM, Vázquez-Nin GH. Necrosis as Programmed Cell Death. In: Cell Death - Autophagy, Apoptosis and Necrosis. InTech.








