EMT, or epithelial-mesenchymal transition, is a biological process in which epithelial cells gain the ability to migrate, allowing them to move away from the tissue in which they originated.1 Under normal conditions, EMT plays a critical role in wound healing and tissue regeneration. However, when cancerous cells hijack this mechanism, it can take on pathological significance and enable tumor spread.
EMT has been linked to both the early and late stages of tumor development. As tumors begin to metastasize, this process helps cancer cells adapt to new environments, enhancing their chances of survival and enabling the formation of aggressive tumors throughout the body. Importantly, EMT can occur before a tumor becomes clinically detectable, making early diagnosis and prevention significantly more challenging.
Adding to the complexity, cancer cells that have undergone EMT often show increased resistance to treatments such as chemotherapy, radiotherapy, and targeted therapies, and are more likely to recur after initial therapy.
 |
Explore related CST antibody sampler kits, which contain reagents to many of the markers mentioned in this blog: • Epithelial-Mesenchymal Transition (EMT) Antibody Sampler Kit #9782 |
|
To better understand and manage these challenges, researchers are focusing on a range of EMT markers. These markers could hold the key to improving early detection, limiting metastasis, and enhancing treatment responses. In this blog, we take a closer look at some of the most promising EMT markers and what they might reveal about cancer progression and treatment resistance.
<Jump to the product list at the end of this post>
How EMT Markers Are Used in Cancer Research
Research is increasingly showing that EMT is not a simple, all-or-nothing process— instead, cancer cells often exist in a spectrum of intermediate states, displaying both epithelial and mesenchymal traits at the same time. This cellular plasticity enables tumor cells to adapt to different environments, evade the immune system, and survive various stresses, including cancer treatments. Because of this dynamic nature, identifying and tracking EMT markers has become a powerful tool in cancer research and can provide insights into how tumors progress, metastasize, and develop resistance to therapy.
1. Changes to Cell Adhesion Proteins
One distinctive hallmark of EMT is the change in levels of two transmembrane proteins: E-cadherin and N-cadherin.
|
|
|
During EMT, E-cadherin—responsible for maintaining tight cell adhesion—is downregulated, while N-cadherin is upregulated. This "cadherin switch" enables cancer cells to detach from the primary tumor and migrate to other parts of the body.2 Both proteins are considered core markers of EMT.
Other adhesion-related proteins are also affected. ZO-1, a peripheral membrane adaptor that links junctional transmembrane proteins to the actin cytoskeleton, is typically reduced during EMT, contributing to increased cellular mobility and metastatic potential.
Similarly, decreased levels of occludin—a transmembrane protein essential for tight junction integrity—can signal weakened adhesion and EMT progression.
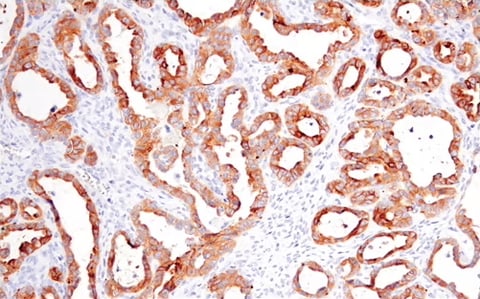
IHC analysis of paraffin-embedded human ovarian clear cell carcinoma using Occludin (E6B4R) Rabbit Monoclonal Antibody #91131.
EpCAM, another transmembrane protein, is highly expressed in many cancer cells. While not an EMT marker in the traditional sense, its elevated presence can also be used to help identify tumor tissue.
2. Disruption in Cytoskeletal Proteins
Cytoskeletal changes are another indicator of EMT. For instance, myofibroblasts normally express a cytoskeletal protein, alpha-smooth muscle actin (SMA), to support wound repair.3 However, cancer cells and cancer-associated fibroblasts (CAFs) also produce large quantities of SMA during EMT, and elevated levels are often linked to a poor prognosis.
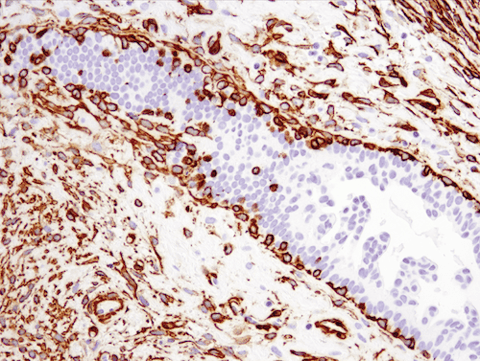
IHC analysis of paraffin-embedded human ductal carcinoma of the breast using recombinant monoclonal antibody α-Smooth Muscle Actin (D4K9N) Rabbit Monoclonal Antibody #19245.
Vimentin, an intermediate filament protein, is another important marker. Its upregulation signals a mesenchymal state and supports increased motility and invasiveness.
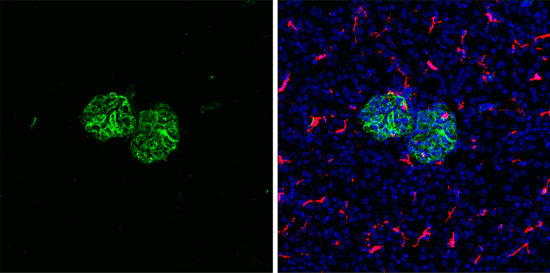
Immunofluorescent (IF) analysis of fixed frozen mouse kidney labeled with recombinant monoclonal antibody Vimentin (D21H3) Rabbit Monoclonal Antibody #5741 (left, green) and co-labeled with F4/80 (BM8.1) Rat mAb (right, red), and ProLong® Gold Antifade Reagent with DAPI #8961 (right, blue).
Fibronectin, a major protein of the extracellular matrix (ECM), is also strongly upregulated during EMT. As a hallmark of the mesenchymal state, fibronectin not only serves as a marker of EMT, but also actively contributes to tumor development, invasion, and metastasis by promoting cell adhesion, migration, and interaction with the tumor microenvironment (TME).
3. Transcription Factors that Regulate EMT
There are a range of transcription factors that suppress epithelial characteristics, allowing the progression of EMT.4
For starters, the repression of E-cadherin expression is driven by factors such as SNAIL, SLUG, and ZEB1. These transcriptional repressors are commonly upregulated during EMT and play a pivotal role in initiating the process.
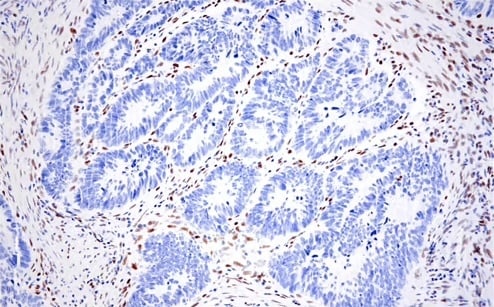
IHC analysis of paraffin-embedded human colon adenocarcinoma using recombinant monoclonal antibody ZEB1 (E2G6Y) Rabbit Monoclonal Antibody #70512.
TWIST, a basic helix-loop-helix (BHLH) transcription factor, also contributes by repressing E-cadherin expression and promoting the mesenchymal phenotype.4
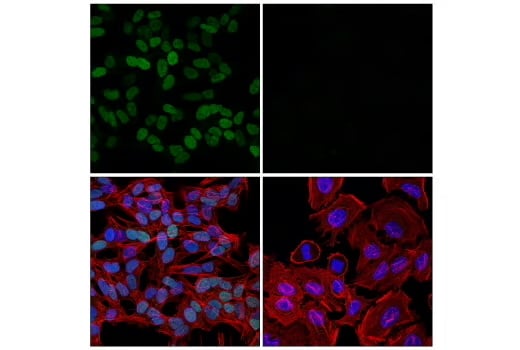
IF analysis of SH-SY5Y cells (left, positive) and HeLa cells (right, negative) using recombinant monoclonal antibody TWIST1 (E5G9Y) Rabbit Monoclonal Antibody #90445 (green), DyLight™ 650 Phalloidin #12956 (red), and DAPI #4083 (blue).
Factors like Oct-4 and Nanog also promote EMT by inducing stem cell-like properties, allowing them to self-renew rapidly and metastasize more effectively.
4. Signaling Proteins that Trigger EMT
EMT can also be triggered by the presence of a number of signaling proteins.
One such protein is DDR2, a receptor tyrosine kinase that is activated by binding to extracellular matrix collagens, leading to changes in gene regulation, including the upregulation of various EMT markers. When DDR2 is silenced, the number of EMT markers is reduced.5
Beta-catenin also plays an important role, particularly in the Wnt signaling pathway, which stimulates EMT.6

IHC analysis of paraffin-embedded human prostate adenocarcinoma using recombinant monoclonal antibody ß-Catenin (D10A8) Rabbit Monoclonal Antibody #8480.
Another contributor is CD31/PECAM1, a cell adhesion molecule commonly associated with the immune response. It is heavily involved in angiogenesis, allowing tumor vascularization and promoting EMT. 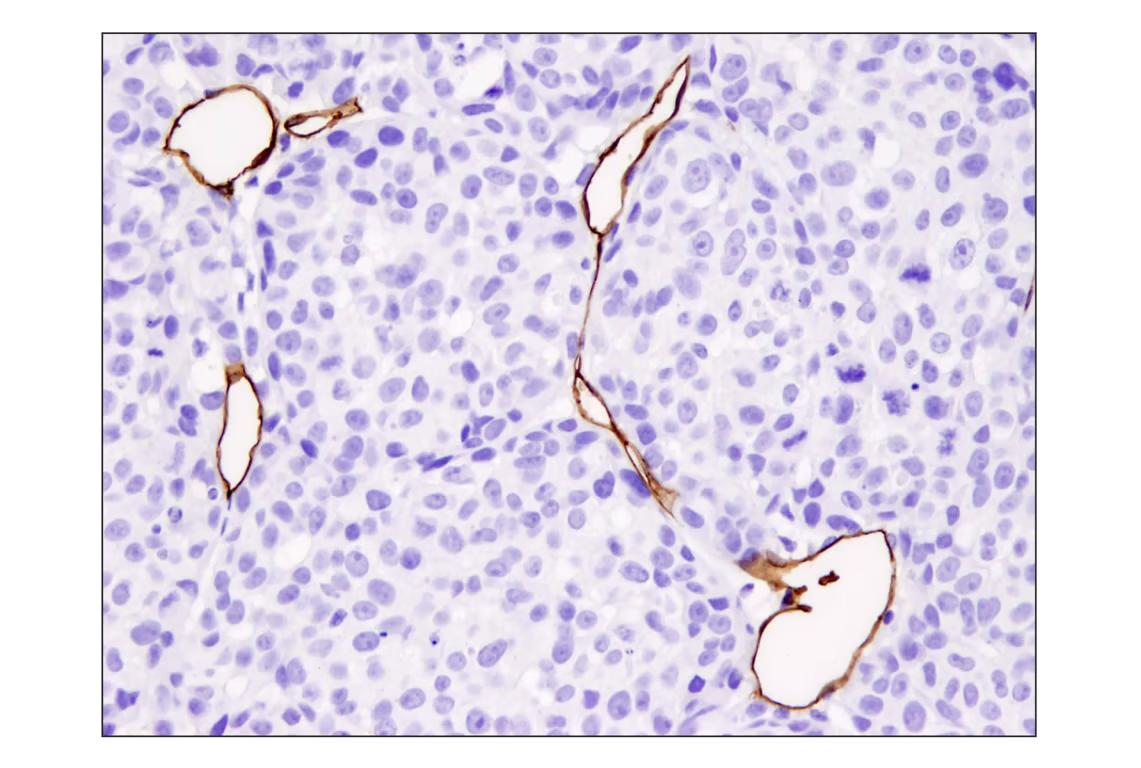
IHC analysis of paraffin-embedded A2058 xenograft using recombinant monoclonal antibody CD31 (PECAM-1) (D8V9E) Rabbit mMonoclonal Antibody Ab.
A Promising Target for Cancer Research
Metastasis accounts for approximately 90% of cancer-related deaths.5 Gaining a clearer understanding of how and why it occurs is essential to improving outcomes for cancer patients.
Each of the markers discussed here can be used to help identify where EMT, an important driver of metastasis, may be taking place. By studying the behavior of these markers and their interactions, researchers aim to develop tools for earlier detection and more effective therapies.
While much more research is needed to fully grasp the underlying mechanisms, every discovery brings us closer to earlier intervention and better long-term outcomes—ultimately improving both survival rates and quality of life for patients.
Select References
- Nisticò P, Bissell MJ, Radisky DC. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol. 2012;4(2):a011908.
- Loh CY, Chai JY, Tang TF, Wong WF, Sethi G, Shanmugam MK, Chong PP, Looi CY. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells. 2019;8(10):1118.
- Sousa AM, Liu T, Guevara O, Stevens J, Fanburg BL, Gaestel M, Toksoz D, Kayyali US. Smooth muscle alpha-actin expression and myofibroblast differentiation by TGFbeta are dependent upon MK2. J Cell Biochem. 2007;100(6):1581-1592.
- Debnath P, Huirem RS, Dutta P, Palchaudhuri S. Epithelial-mesenchymal transition and its transcription factors. Biosci Rep. 2022;42(1):BSR20211164.
- Kim D, You E, Jeong J, et al. DDR2 controls the epithelial-mesenchymal-transition-related gene expression via c-Myb acetylation upon matrix stiffening. Sci Rep. 2017;7:6847.
- Xue W, Yang L, Chen C, Ashrafizadeh M, Tian Y, Sun R. Wnt/β-catenin-driven EMT regulation in human cancers. Cell Mol Life Sci. 2024;81(1):79.

 IHC analysis of paraffin-embedded human gastric carcinoma using
IHC analysis of paraffin-embedded human gastric carcinoma using 




