As a researcher, your experiments are designed to ask new questions and pursue scientific knowledge by building on current research. How does this dynamic affect experimental design when you're comparing which antibodies and reagents to use? Gathering as much information about your target molecule before you begin will help ensure the success of your immunoassay.
In the final Antibody Essentials blog, we'll build on the learnings from the first four posts in the series to help you determine which questions to ask as you design your experiments, select targets, and choose the right antibody reagents.
Table of Contents
Understanding Your Target Protein
In hypothesis-driven research, you’ll start with an idea about a particular biological function, activity, or mechanism in your experimental model. This hypothesis may come from the results of a previous experiment, a literature review, or from discussions with collaborators. Refining your hypothesis and experimental design, including target and antibody selection, often go hand-in-hand. The better you understand your target protein's biology, the more informed your antibody selection will be.
The target protein characteristics you should consider include its expression level and subcellular localization, as well as its structure, stability, and homology to related proteins. It’s also important to consider whether your protein undergoes any post-translational modifications (PTMs) or is the target of upstream signaling events. These factors will provide valuable insights into its overall biological context and can guide your experimental design and antibody selection.
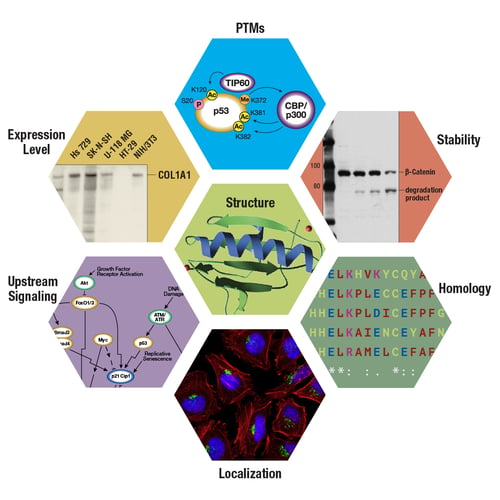
Figure 1. The characteristics of the target protein may influence experimental design and protocol optimization decisions. Depending on the target, model system, application, and experimental aim, some of the target characteristics shown here may merit more consideration than others.
Consulting open-access resources such as Uniprot or the Human Protein Atlas and reading the existing literature are also important ways to learn about your target protein. Resources on signal transduction, such as CST cellular signaling pathway diagrams and PhosphoSitePlus, a curated database of protein modifications and systems biology, can provide important context about the biology of your target protein. This information can also be useful for selecting experimental controls, designing other components of your experiment, such as what activators or inhibitors to use to perturb signaling activities, or identifying the next target for a follow-up experiment.
Finally, colleagues and peers in your network can also be a source of information. Check antibody review sites to see researcher feedback regarding antibody performance and to look for advice on optimizing different immunoassay protocols.
%20sc-7480%20vs%20CST%20Bax%20Antibody.webp?width=250&height=202&name=Santa%20Cruz%20(B-9)%20sc-7480%20vs%20CST%20Bax%20Antibody.webp) |
Blog: Watch Your Bax: How a Faulty Antibody May Have Cost Millions in Flawed Research |
Using Search Engines to Find Antibodies
After compiling a list of experimental targets, you’ll likely go to Google or another search engine using your target of interest and desired application as search terms.
For example, if you're studying a process that activates necroptosis (a form of regulated cell death) in a cultured mouse cell line using immunofluorescence-based microscopy, your experimental design will likely include primary antibodies for the key proteins involved. During necroptosis, the RIP1 and RIP3 kinases form a complex and phosphorylate each other, leading to the phosphorylation and activation of MLKL, which acts as the effector in necroptosis. Therefore, you might search for “RIP1 antibody," "RIP3 antibody," or “MLKL antibody”, and include terms like “mouse,” “monoclonal,” or “phospho” in your query.
The results of each antibody search will include product pages from various antibody manufacturers. Sometimes, search engines will return antibodies that meet some, but not all, of your requirements, so you'll need to examine each product page for specific details. You'll want to check which validation experiments were performed, and look for immunofluorescence data to ensure the antibody was tested in your intended application.
Based on this information, you’ll start to narrow down your list. You can also consider using the “filtering” function in the antibody search page on cellsignal.com or on a reagent-focused search site such as CiteAb or Biocompare to narrow your search. But either way, your next step is to collect more information.
Target Species Reactivity
Examine each antibody product page for information that supports the target species reactivity. Recall the distinction between target species reactivity and antibody host species, keeping in mind that the phrase “RIP3 mouse antibody” might return mixed search results, including mouse host antibodies that don’t necessarily react with the mouse RIP3 protein target.
Check the listed species reactivity and look for validation data performed with cells or tissues from the desired species to support the antibody’s species reactivity.
Application
Your initial search results may include a number of antibodies with the desired target reactivity and species reactivity, some of which have been validated in your desired application, but others that have not. Even if you include “immunofluorescence” or the name of another application as a search modifier, it’s a good idea to check the data on the product page to ensure that validation testing was performed in your intended application. Keep in mind that validation in one application does not necessarily mean an antibody will work in another application—validation testing must be performed in each application independently to ensure antibody specificity, sensitivity, and reproducibility.
When you're checking the antibody's intended application, look for details about the experimental design and protocol used during validation testing. What cells or tissues were used? Were they stimulated with a ligand or treated with an inhibitor? Were the appropriate controls included? How were the samples prepared and processed? The answers to these questions can help you decide whether the antibody is likely to work with your samples and protocol, or if you will need to consider adding your own validation tests before scaling up your experiments.
Keep in mind that no single assay is sufficient to determine the validity of an antibody—even when using knockout of the target molecule. Confirming that an antibody is both specific and sensitive depends on the application and protocol being used, the type and quality of sample being analyzed, and the inherent biophysical properties of the antibody itself. The performance of an antibody in one application, such as immunofluorescence with a cell culture model, doesn’t necessarily predict its specificity or performance with another application and another protocol, such as immunohistochemistry with paraffin-embedded tissue samples. This is because sample preparation can affect the epitope's structure and availability, and different binding conditions can alter the strength of the antibody-antigen interaction.
In the Hallmarks of Validation video below, you can learn more about antibody performance and how CST validates antibodies. It provides an overview of the different strategies we use, which you can adapt for your own validation experiments.
Antibody Host Species, Isotype, & Subclass Considerations
Why should you note the host species and isotype of the target antibody? Many immunoassays use a conjugated secondary antibody to generate a signal after the primary antibody has bound to its target. This approach is used for chemiluminescent detection in western blot, and fluorescence-based amplification and detection in immunocytochemistry, flow cytometry, and other assays. This process requires a secondary antibody that recognizes the primary antibody, which is where the host species and isotype of the primary antibody become important.
Some secondary antibodies recognize multiple isotypes from one species (such as mouse), while others recognize only one isotype (such as mouse IgG) or subclass (such as mouse IgG2a). Matching primary and secondary antibodies also enables the design of multiplexed experiments that use pairs of primary and secondary antibodies with different isotype and fluorophore combinations. Alternatively, directly conjugated primary antibodies can be considered; if you go this route, you would ideally want to see validation data generated using the specific antibody conjugate you will be using.
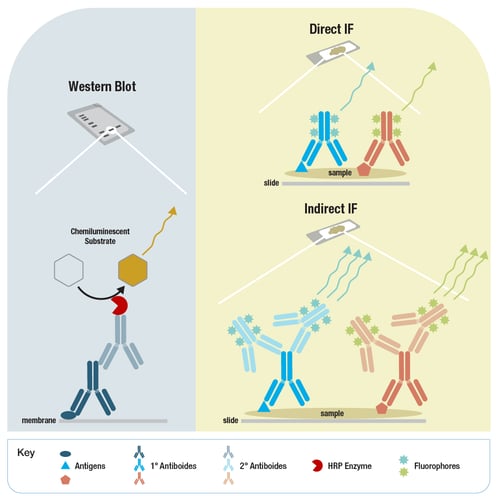 Figure 2. Secondary antibodies are conjugated to an enzyme such as HRP for use in chemiluminescent detection for western blot (left), or to fluorophores with distinct emission wavelengths for use in indirect immunofluorescence (lower right).
Figure 2. Secondary antibodies are conjugated to an enzyme such as HRP for use in chemiluminescent detection for western blot (left), or to fluorophores with distinct emission wavelengths for use in indirect immunofluorescence (lower right).
Avoiding Cross-Reactivity
The antibody host species is also important in relation to the species that your sample material came from. In immunohistochemistry, mouse-on-mouse cross-reactivity can arise when anti-mouse secondary antibodies bind to endogenous mouse immunoglobulins in the sample, resulting in unwanted background signal. This can limit the utility of mouse-derived antibodies when working with certain mouse tissue samples. Similar background staining can also occur if secondary antibodies bind nonspecifically in the sample, making it vital that you choose well-validated secondaries and include relevant controls to avoid generating misleading results.
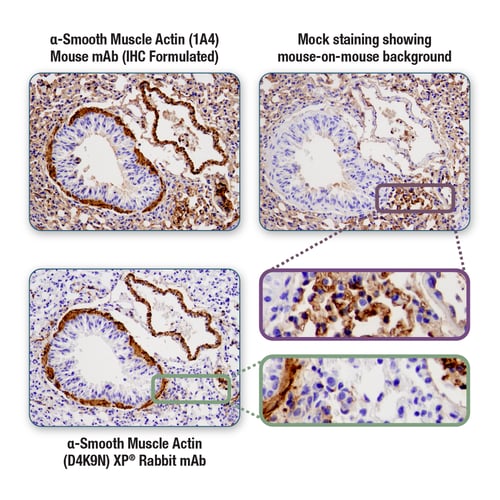 Figure 3. Immunohistochemical analysis of mouse lung tissue, performed with a mouse host antibody to α-Smooth Muscle Actin and anti-mouse secondary detection reagent (upper left), or mock-stained with anti-mouse secondary detection reagent alone (upper right). The latter indicates the presence of mouse-on-mouse background staining. By switching to a rabbit host antibody for the same target and anti-rabbit secondary detection reagent (lower left), mouse-on-mouse background is removed.
Figure 3. Immunohistochemical analysis of mouse lung tissue, performed with a mouse host antibody to α-Smooth Muscle Actin and anti-mouse secondary detection reagent (upper left), or mock-stained with anti-mouse secondary detection reagent alone (upper right). The latter indicates the presence of mouse-on-mouse background staining. By switching to a rabbit host antibody for the same target and anti-rabbit secondary detection reagent (lower left), mouse-on-mouse background is removed.
Antibody Clonality: Monoclonal, Polyclonal & Recombinant
We discussed the advantages and disadvantages of monoclonal vs polyclonal antibodies in the previous Antibody Essentials blog. For now, we'll just reiterate that when available, it’s recommended that you choose a recombinant monoclonal antibody for your research to benefit from the consistent performance.
- Polyclonal antibodies: A mix of antibodies that bind to multiple epitopes, providing a strong signal but with higher lot-to-lot variability and the potential for cross-reactivity.
- Monoclonal antibodies: Derived from a single B-cell clone, monolonals are highly specific and bind to only a single epitope, resulting in minimal cross-reactivity and low lot-to-lot variation.
- Recombinant antibodies: Monoclonal antobodies produced using in vitro genetic engineering. Benefits include animal-free manufacturing, superior lot-to-lot consistency, and scalable supply.
However, if you’re detecting a novel target, a polyclonal may be your only option until a monoclonal has been developed. If this turns out to be the case, you may decide to perform your initial studies with a polyclonal antibody, subsequently deciding between a mouse monoclonal or a rabbit monoclonal when these become available. Both mouse and rabbit monoclonals provide many advantages compared to polyclonals, meaning that choosing between them will largely be dictated by the unique requirements of your immunoassay.
Antibody Production Considerations
While most commercially available antibodies are supplied as a purified preparation in an aqueous buffer, you might encounter less purified preparations, such as a crude serum isolated from blood. Alternatively, if hybridomas are used for monoclonal production, the antibodies may be collected from cell culture media or from ascitic fluid collected from mice that have been injected with hybridomas. These can be problematic if you plan to conjugate the antibody to an enzyme or a fluorescent dye, or if you wish to perform an experiment such as an immunoprecipitation, since the antibody concentration is typically low. These crude preps can also contain other proteins or components that could interfere with antibody conjugation or have other unintended consequences on your assay, meaning you would need to consider if additional purification is necessary.
While it’s possible to perform a buffer exchange and concentrate the antibody in your own lab, this is often time-consuming and can be highly prone to variability. If you work in a lab or core facility that uses specialized or high-throughput immunoassay formats, you may consider carrier-free or custom formulations or bulk quantity purchases to suit your needs. For the majority of bench scientists, purchasing standard formulation purified antibodies provides enough options to probe the targets they're interested in using common applications and fairly standard protocols.
Antibody Specificity & Sensitivity
In the first Antibody Essentials blog, we defined antibody specificity and sensitivity. Both are crucial when you’re choosing an antibody to support your research.
 |
Blog: Do You Trust Your Research Antibody? |
Antibody Specificity for the Target Protein
Specificity ensures your antibody recognizes only your target protein without cross-reacting with other similar proteins. This is especially important if you’re detecting a single protein within a family of closely related proteins—you’ll want to make sure that the antibody you choose recognizes only the protein you’re interested in, without cross-reacting with other family members. While sequence alignment can suggest homology, the only way to ensure antibody specificity is to review testing data. A trusted antibody manufacturer will have generated these data during antibody validation, eliminating the need for you to perform cross-reactivity testing yourself. They may have determined whether the antibody can recognize the equivalent protein target in other species. While cross-species reactivity can be useful in certain situations—for instance, if you’re comparing the response to a drug in both human and mouse cell lines—in many other cases, it should be avoided.
For example, if you’re planning to use the antibody to monitor the expression levels of an engineered protein in a cell line, you might significantly overestimate protein expression if the antibody also detects the endogenous protein expressed by the host cell.
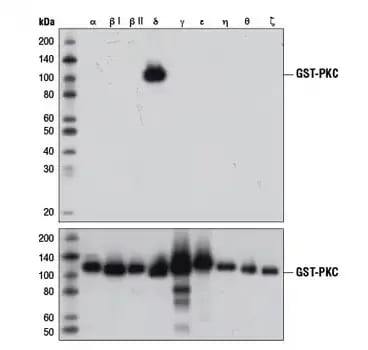
Figure 4. Isoform-specific detection of PKCδ. Bacterially expressed, GST-tagged, purified PKC isoforms were loaded into the lanes indicated for SDS-PAGE and western blot analysis using a PKCδ antibody (upper) or GST antibody (lower).
Antibody Sensitivity for the Target Protein
Finally, you’re going to want to consider antibody sensitivity, which brings us full circle back to understanding your antigenic target. If your protein is of low abundance, you’ll want to compare several different antibodies to see which produces the strongest signal in your immunoassay while retaining specificity.
For phospo-specific antibodies, you’ll want to see confirmation that the antibody is specific for your target when phosphorylated at a particular residue and that it is sensitive enough for your application. Consider which cell types are known to express the phosphorylated protein, and whether a stimulus will be required to detect it.
If you’re planning to use a directly conjugated primary antibody in an immunoassay that has a fluorescent readout, you’ll want to determine if your chosen antibody is available conjugated to a bright fluorophore that is detectable by your instrumentation, and consider your target's abundance.
Consulting with Your Peers
Searching the published literature and digging into the materials and methods sections and supplemental figures of research papers may yield more information on antibodies that you are considering. Seeing that a particular antibody performed in your desired application, with similar sample materials such as cell line or tissue, can help instill confidence that you are making the right choice. However, it is important to remember that inaccurate or incomplete information in the literature has been a contributing factor in the reproducibility crisis, so always maintain a critical eye. Of course, colleagues in your lab, down the hall, or in your network can be a source of information as well. They may have done their own hands-on comparison of different antibodies or may have optimized an antibody for the same experimental model you plan on using. You can also check antibody review sites and even social networks, where researchers sometimes provide feedback regarding antibody performance. Again, the details matter, and a healthy dose of skepticism is a good idea until you can be confident the antibody is validated and working in your hands.
We hope we’ve provided you with some useful information to help choose antibodies to support your research, but if you take away only two messages from Antibody Essentials, it’s these:
-
No single assay is sufficient to determine the validity of an antibody.
-
Think critically about the antibodies you are planning to use, as if you were producing and validating them yourself.
Keeping these principles at the front of your mind will go a long way in avoiding setbacks in your research, and ensure you can get the best out of these critical and valuable research reagents.
Read the entire Antibody Essentials series:
- Antibody Essentials Part 1: Antibody Basics
- Antibody Essentials Part 2: Antibody Diversity and Classification
- Antibody Essentials Part 3: How Antibody Technologies Evolved
- Antibody Essentials Part 4: Polyclonal vs Monoclonal Antibodies
- Antibody Essentials Part 5: Important Considerations for Antibody Selection
20-FLE-62535







