Over the last 50 years, laboratories have been able to demonstrate through experimentation the processes contributing to cell death. Early discoveries focused on morphological features of cell death and classifications into apoptosis and necrosis. Since then, there have been many more discoveries regarding the programmed cellular pathways contributing to apoptosis.
We’ve come to understand more about the basic machinery of apoptotic programmed cell death through proteins like caspase 3, bcl-2, and Fas. While cell death might seem simple, there are many more signaling pathways that contribute to cell death than initially suspected.
Regulated Necrotic Cell Death: Necroptosis vs Pyroptosis
Over the last few years, exciting advances have been made in the study of regulated necrotic cell death playing widespread physiopathological roles with therapeutic implications. Necroptosis and pyroptosis represent two of these regulated pathways that share morphological features of necrosis, including cell swelling, plasma membrane pore formation, and membrane rupture. These processes trigger an inflammatory response with the release of a number of damage-associated molecular patterns (DAMPs) such as HMGB1 and inflammatory cytokines like interleukin-1β (IL-1β) and IL-18.
Necroptosis
Necroptosis is a cell defense pathway that is activated under conditions in which apoptosis is inhibited. It requires the activation of the RIPK3 kinase, which phosphorylates the MLKL pseudokinase. MLKL phosphorylation at Ser358 (Ser345 in mouse) leads to oligomerization of MLKL and the formation of a pore-forming complex. MLKL pores form cation channels that lead to further membrane rupture and secretion of DAMPs. RIPK3 activation is triggered through several RIP homotypic interaction motif (RHIM) domain interactions, including RIPK1, TRIF, and ZBP1/DAI, and results in the phosphorylation of RIPK3 at Ser227 (Thr231/Ser232 in mouse). Canonical necroptosis signaling is mediated by RIPK1 following stimulation by members of the TNF family and results in the autophosphorylation of RIPK1 (including Ser166). This pathway can be inhibited by necrostatins (Nec-1, Nec-1s), direct inhibitors of RIPK1.
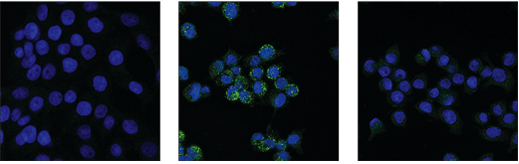 IF analysis of HT-29 cells, untreated (left), pre-treated with Z-VAD (20 μM, 30 min) followed by treatment with SM-164 (100 nM) and Human Tumor Necrosis Factor-α (hTNF-α) (20 ng/mL, 6 hr; center), or pre-treated with Z-VAD followed by treatment with SM-164 and hTNF-α and post-processed with λ-phosphatase (right), using Phospho-RIP3 (Ser227) (D6W2T) Rabbit mAb #93654 (green).
IF analysis of HT-29 cells, untreated (left), pre-treated with Z-VAD (20 μM, 30 min) followed by treatment with SM-164 (100 nM) and Human Tumor Necrosis Factor-α (hTNF-α) (20 ng/mL, 6 hr; center), or pre-treated with Z-VAD followed by treatment with SM-164 and hTNF-α and post-processed with λ-phosphatase (right), using Phospho-RIP3 (Ser227) (D6W2T) Rabbit mAb #93654 (green).
Alternatively, RIPK3 is activated by innate immune responses, including Toll-like receptor (TLR) recruitment of TRIF or by DNA virus-induced activation of ZBP1/DAI. Activation of necroptosis under these circumstances represents an alternative cell death mechanism that can be activated in cases where the pathogens find ways to subvert apoptosis. Apoptosis inhibits necroptosis through caspase-8-mediated cleavage of RIPK1 and RIPK3. DAMPs released during necroptosis lead to an inflammatory response including activation of pyroptosis.
Pyroptosis
Pyroptosis shares some similarities to necroptosis, but while necroptosis is thought to be a secondary cell death response to situations where apoptosis is inhibited, pyroptosis is generally a primary response to infectious organisms. Pyroptosis is induced in cells of the innate immune system, such as monocytes, marcrophages, and dendritic cells in the presence of pathogen-associated molecular patterns (PAMPs) expressed on microbial pathogens or by cell-derived DAMPs. Canonical induction of pyroptosis requires activation of caspase-1, which cleaves and activates inflammatory cytokines like IL-1β and IL-18. In addition, caspase-1 cleaves the pore-forming protein gasdermin D (GSDMD).
Explore the interactive Necrotic Cell Death Pathway diagram
Upon cleavage, the N-terminal fragment of gasdermin D oligomerizes to form a pore similar to, but larger than, MLKL, allowing secretion of inflammatory DAMPs and cytokines. In this process, caspase-1 is activated by a variety of inflammasome complexes, typically consisting of a cytosolic-pattern recognition receptor (PPR; a nucleotide-binding domain and leucine-rich repeat [NLR] or AIM2-like family members), an adaptor protein (ASC/TMS1), and pro-caspase-1. Distinct inflammasome complexes can recognize distinct PAMPs and DAMPs to trigger pyroptosis. The best-characterized pathway triggered by the NLR, NLRP3, occurs through a two-step process. The first step is a priming signal, where NF-kB is activated to induce the expression of a number of inflammasome components including NLRP3, pro-IL-1β, and pro-IL-18. In the second activation step, caspase-1 is activated and gasdermin D and cytokines are proteolytically activated.
Necrosis Signaling Pathways and Disease
While regulated necrosis signaling pathways are frequently studied in the context of infectious diseases, they are proving to have widespread pathological significance, including neurodegeneration, autoimmune disease, cancer, metabolic diseases, cardiovascular disease, inflammation in the liver, pancreas, kidney, and intestine, as well as other chronic inflammatory diseases. Components of these pathways can serve as key biomarkers as well as potential therapeutic targets.
Currently, therapeutic strategies have been realized targeting IL-1b and its receptor for the treatment of arthritis and other inflammatory disorders. However, as these approaches can lead to general immune suppression, more targeted approaches to inhibiting the inflammasome, including NLRs and inflammatory caspases, may provide additional, widely applicable therapeutics. Inhibitors for necroptosis have also been identified that directly target RIPK1, RIPK3, and MLKL. Clinical studies are already being performed for RIPK1 inhibitors, which may be used for neurodegenerative and inflammatory diseases.
Together, these pathways offer tremendous promise for the understanding and treatment of disease.
To learn more about the mechanisms, morphology, and key proteins involved in many types of cell death, download the Researcher's Guide to Mechanisms of Cell Death.
Necroptosis and Pyroptosis Antibody Sampler Kits
Select References:
- Shan B, Pan H, Najafov A, Yuan J. Necroptosis in development and diseases. Genes Dev. 2018;32(5-6):327-340. doi:10.1101/gad.312561.118
- Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26(1):99-114. doi:10.1038/s41418-018-0212-6
- Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42(4):245-254. doi:10.1016/j.tibs.2016.10.004
- Weinlich R, Oberst A, Beere HM, Green DR. Necroptosis in development, inflammation and disease. Nat Rev Mol Cell Biol. 2017;18(2):127-136. doi:10.1038/nrm.2016.149
- Zakeri Z, Lockshin RA. Cell death: history and future. Adv Exp Med Biol. 2008;615:1-11. doi:10.1007/978-1-4020-6554-5_1






