Most people will either be directly or indirectly affected by a cancer diagnosis at some point in their lifetime. World Cancer Day, created in 2000 and organized by the Union for International Cancer Control, is held every year on February 4th to raise worldwide awareness, improve education, and catalyze investment in cancer research in order to bring us closer to a future without cancer. Cell Signaling Technology is proud to play our part, supporting World Cancer Day by providing trustworthy, stringently validated reagents, kits, and applications that drive our oncology knowledge forward all over the world and by engaging with the community to raise funding for critical outreach and research programs.
This year, World Cancer Day is focused on closing the gap in cancer care so all cancer patients can get high-quality care regardless of their income, education, geographical location, ethnicity, and lifestyle. Hundreds of events and fundraisers will take place around the world to support the development of life-saving cancer prevention, diagnosis, and treatment options.
The Cancer Treatment Landscape
In 2020, there were approximately 18 million cases of cancer diagnosed worldwide, of which 1.8 million cases and 606,520 deaths were reported in the United States alone. Breast cancer was the most commonly diagnosed type of cancer, with 2.26 million new cancers reported worldwide, followed by lung cancer, colon and rectum cancer, prostate cancer, non-melanoma skin cancer, and stomach cancer. Death rates have steadily declined at a rate of approximately 2.3% for men and 1.9% for women over the years, thanks to a better understanding of the disease, early diagnostic tools, and improved treatment options. However, not all cancer deaths are on the decline. Deaths attributed to pancreatic cancer and melanoma are on the rise.
So, what may be causing the discrepancy? Education about prevention and early detection certainly plays a significant role. Scientific breakthroughs that lead to improved treatment modalities also contribute; however, cancer is a disease that can affect almost any organ or tissue, and the mechanisms involved in disease initiation and progression are different even within a specific type of cancer. Therefore, finding one “silver bullet” that can eradicate all cancers will be challenging. Additionally, cancers can become resistant to specific treatments over time, as the cancer cells and the tumor microenvironment (TME) continue to change. Thus, clinicians need a toolbox with a variety of therapeutic treatments that utilize different approaches when attacking cancer cells.
Fortunately, the continued efforts of programs like World Cancer Day and the Cancer Moonshot are marshaling a community of patients, advocates, researchers, and clinicians to improve cancer survival rates. The Cancer Moonshot initiative has the goal of reducing cancer deaths by half by 2047 and improving the quality of life for cancer patients and survivors alike. The National Cancer Institute (NCI) has also been allocated $7.3 billion from the Consolidated Appropriations Act, 2023. Of that, $216 million is designated for the NCI component of the Cancer Moonshot.
Unleashing the Immune System to Treat Cancer
The past decade has seen a flurry of new therapeutic approaches that have significantly changed the cancer treatment landscape. Immunotherapies harness a patient’s own immune system to fight cancer. T cells can be capable of killing tumor cells, but upregulate suppressive molecules, referred to as immune checkpoints, in the context of cancer that limit their activity. Many of the approved cancer immunotherapies are antibodies that inhibit the function of the immune checkpoints PD-1 and CTLA-4. These therapies can be extremely effective, but only in a subset of patients.1 Therefore, there is substantial effort underway to identify additional immunotherapy targets and to combine therapies targeting PD-1 with other therapies.
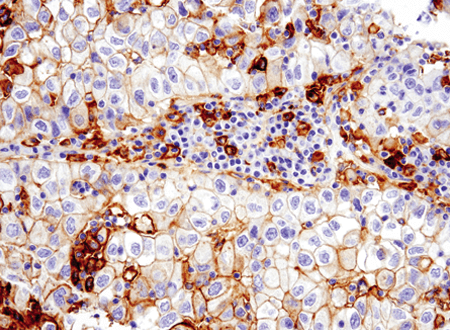
Immunohistochemical analysis of paraffin-embedded human non-small cell lung carcinoma using PD-L1 (E1L3N) #13684.
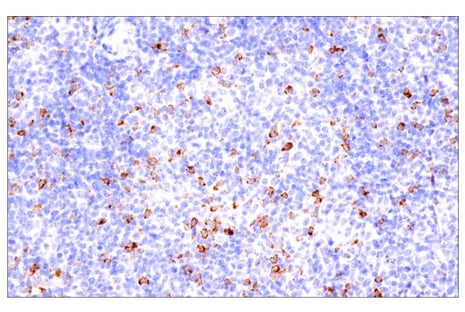 Immunohistochemical analysis of paraffin-embedded human B cell non-Hodgkin lymphoma using CTLA-4 (E2V1Z) #53560.
Immunohistochemical analysis of paraffin-embedded human B cell non-Hodgkin lymphoma using CTLA-4 (E2V1Z) #53560.
Recently, the US Food and Drug Administration approved a new T cell immune checkpoint inhibitor targeting LAG3 (relatimab) in combination with the PD-1 inhibitor, nivolumab for melanoma. The combination of nivolumab and relatimab was as effective as the combination of nivolumab and ipilimumab, a CTLA-4 inhibitor, but with substantially fewer side effects.2,3
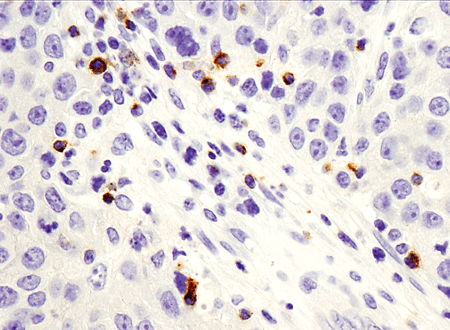 Immunohistochemical analysis of paraffin-embedded human breast ductal carcinoma using LAG3 (D2G4O™) XP®#15372.
Immunohistochemical analysis of paraffin-embedded human breast ductal carcinoma using LAG3 (D2G4O™) XP®#15372.
Adding to the Immunotherapy Toolbox Using Spatial Biology and Single-Cell Analysis
Researchers are also looking at ways to trigger other immune cell types within the TME.4 Resistance to checkpoint inhibitors can occur due to a “cold tumor” microenvironment. A better understanding of how all the different cell types found in the TME interact and influence each other can lead to strategies that will turn cold tumors "hot." However, deconstructing the interplay between malignant cells and the TME is challenging due to the heterogeneity in the TME as well as within the tumor.4,5 The emergence of spatial biology tools in combination with single-cell techniques can be used to unravel this complex ecosystem.
Single-cell analysis can help researchers understand the differences between malignant cells in the middle of a tumor compared to the edges and how that affects treatment efficacy and resistance, as well as how signals can emanate from the TME and influence tumor behavior. Tumor cells do not live in a vacuum—there is a rich and complex network of signals regulating tumor behavior. Spatial biology can elucidate how different cells in the tumor and TME work together to initiate and drive disease progression, predict therapeutic response, and potentially shed light on how resistance emerges. These investigations can enable the development of immuno-oncology therapeutics that utilize other immune cells, like dendritic cells, natural killer cells, B cells, and neutrophils. Using a cocktail of immunotherapies that take advantage of a combination of immune cells to promote an anti-tumor response can potentially overcome immune resistance to give patients long-term remission outcomes.7
Understanding how interactions between tumor cells and the TME impact disease progression may also lead to new strategies for leveraging cell death, senescence, and metabolic reprogramming to develop more effective, more specific, and less toxic oncology therapeutics. These studies will be aided by using readouts like p16 INK4A; a senescence and cervical cancer biomarker.
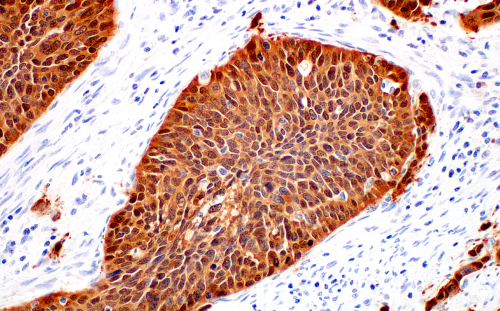 Immunohistochemical analysis of paraffin-embedded human squamous cell carcinoma of the cervix using p16 INK4A (BC42) Mouse mAb #68410.
Immunohistochemical analysis of paraffin-embedded human squamous cell carcinoma of the cervix using p16 INK4A (BC42) Mouse mAb #68410.
Combining T-Cell Therapies and Vaccines to Treat Solid Tumors
Chimeric antigen receptor (CAR) T-cell therapies are another exciting approach that primes a cancer patient’s immune system. CAR-T therapies essentially reprogram a patient’s own T cell to recognize a tumor antigen expressed by the tumor cell. Used primarily for blood cancers, CAR-T cells are a very effective and personalized approach that offers durable remission for patients who have not responded to other types of treatment. However, it is often used as a last resort, in part because of the high price tag associated with developing and validating a CAR-T cell that is specific to a patient. Process improvements to streamline validation, like utilizing a panel of versatile detection reagents developed by CST to confirm the CAR expression, may bring costs down and help close treatment access gaps.
 Immunohistochemical analysis of paraffin-embedded human serous papillary carcinoma of the ovary using Claudin-6 (E7U2O) #18932.
Immunohistochemical analysis of paraffin-embedded human serous papillary carcinoma of the ovary using Claudin-6 (E7U2O) #18932.
Other challenges include toxicities that can occur with treatment, changes to CAR-T cell function in response to interactions with the TME, limited tumor infiltration, and limited efficacy against solid tumors due to low tumor antigen expression and heterogenous antigen expression in the tumor.7 However, investigators are continually identifying novel antigens and developing new strategies to overcome these challenges.
Blog: Antibody Validation for IHC: Finding a Claudin-6 mAb Clone
For example, clinical trials using CAR-T cells that target Claudin-6 have yielded promising results. Claudin-6 is a novel oncofetal antigen expressed in solid tumors such as ovarian cancer, sarcoma, testicular cancer, endometrial cancer, and gastric cancer. Not only were side effects manageable, but researchers found that treating patients with the Claudin-6 CAR-T cells in combination with a CLDN6-encoding mRNA vaccine (CAR-T Cell Amplifying RNA Vaccine—CARVac) promoted CAR-T proliferation and expansion to improve T cell persistence and infiltration.
Vaccines to Prevent and Treat Cancer
The use of mRNA technology has accelerated the speed at which vaccines can be developed, opening new possibilities as to how vaccines can be used to amplify immunotherapy and CAR-T efficacy, as described above. Currently, there are clinical trials in progress evaluating the efficacy of immunotherapy/CAR-T treatments alone and in combination with a vaccine for advanced melanoma, colorectal cancer, and non-small cell lung cancer, to name a few.8
Cancer vaccines can also be used to lower the risk of certain viral infections that lead to cancer development. The human papillomavirus virus (HPV) vaccine, along with routine pap smears for early detection, is making the elimination of cervical cancer a possibility—as long as young girls around the world of all levels of economic status get access. Other viruses that can increase the chance of developing cancer include Epstein-Barr (Burkitt lymphoma, stomach cancer), hepatitis B and hepatitis C (liver cancer), HIV (Kaposi sarcoma, cervical cancer, some kinds of non-Hodgkin lymphoma), and Human Herpes virus 8 (Kaposi sarcoma and primary effusion lymphoma).
We’re Playing Our Part to Help Stop Cancer
When deciphering the multiple cellular events that drive cancer progression, you don’t want low-quality reagents to slow you down or, worse yet, lead you to the wrong conclusion. CST is proud to have offered high-quality antibodies you can trust for over 20 years. And don’t just take our word for it. CiteAb has consistently ranked CST as a top provider of antibodies against a wide range of cancer targets—for example, CST was the antibody supplier with the most citations for products used in cancer research in 2022. We also put our scientific expertise to work for you, developing validated IHC antibodies and reagents against the latest cancer biomarkers.

Our support also extends to the community and members of the CST family impacted by cancer. This past year, CST employees were inspired by Linda, the mother of Associate Scientist Jordan Hirschfield, to form the “Signaling For A Cure” team and participated in the Everyday Amazing 5K Walk/Race sponsored by the Mass General Cancer Center. The team walked to celebrate Linda’s 5th year of remission after conquering stage III lung cancer and raised thousands of dollars to support cancer research and create a brighter future for cancer patients and their families.
Championing Cancer Breakthroughs
Cancer is a debilitating disease that will affect most people in their lifetime. World Cancer Day champions scientific breakthroughs, community education, and outreach that will improve the quality of life and survival outcomes for cancer patients. At CST, we’re proud to do our part to bring us closer to a cancer-free world. Check out the World Cancer Day website to learn how you can get involved as well!
Select References
-
Seliger B. Basis of PD1/PD-L1 Therapies. J Clin Med. 2019;8(12):2168. Published 2019 Dec 8. doi:10.3390/jcm8122168
- LAG-3 inhibitors: A new type of immunotherapy, https://www.mdanderson.org/cancerwise/lag-3-inhibitors--a-new-type-of-immunotherapy.h00-159544479.html
- Huo JL, Wang YT, Fu WJ, Lu N, Liu ZS. The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front Immunol. 2022;13:956090. Published 2022 Jul 26. doi:10.3389/fimmu.2022.956090
- Shi Y, Tomczak K, Li J, Ochieng JK, Lee Y, Haymaker C. Next-Generation Immunotherapies to Improve Anticancer Immunity. Front Pharmacol. 2021;11:566401. Published 2021 Jan 11. doi:10.3389/fphar.2020.566401
- Coulson A. How is spatial transcriptomics influencing cancer research and diagnostics?. Biotechniques. 2022;73(5):215-217. doi:10.2144/btn-2022-0111
- Fu T, Dai LJ, Wu SY, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J Hematol Oncol. 2021;14(1):98. Published 2021 Jun 25. doi:10.1186/s13045-021-01103-4
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021 Apr 6;11(4):69.
-
MRNA medicines we are currently developing. Moderna. https://www.modernatx.com/research/product-pipeline.
-
Technologies for customized treatment approaches. BioNTech. https://www.biontech.com/int/en/home/pipeline-and-products/pipeline.html. 22-CAN-31278






