So you've set the timer to five minutes for the first of three TBST washes for your western blot membrane. Now what? Sure, you could check your email or social media for the 30th time before lunch. Or you could do something informative, like check out a CST Tech Tips video!
This is a new short video series featuring the same scientists who develop and validate CST antibodies, here to offer insights and protocol tips.
Selecting a Blocking Buffer Protein for Your Western Blot
This CST Tech Tips video examines how your choice of membrane-blocking protein—nonfat dry milk vs bovine serum albumen (BSA)—can affect your western blot results.
In the video, CST Product Scientist Srikanth Subramanian, PhD, goes through the considerations for membrane-blocking protein selection, including whether the phosphatases in milk will affect your signal.
We'll be posting more Tech Tips videos here on the blog, but if you have a YouTube account, make sure to check out the Tech Tips playlist and subscribe, so you'll be notified when fresh videos are uploaded.
Video Transcript:
What protein should I use to block a transfer membrane for a western blot? My name is Srikanth, I'm a Product Scientist at Cell Signaling Technology, and this is CST Tech Tips.
In regards to western blotting, a common question we always get is, what should I use, milk or BSA to block? So the purpose of the blocking step is to reduce the amount of background due to non-specific bonding. Now, BSA is only made up of one protein, BSA at 60 kDa, whereas milk is made up of many proteins, all of various sizes. So you get a much better chance to reduce more of the background banding.
We recommend that you use 5% milk in TBST, shaken for one hour at room temperature, to block all of our non-conjugate primary antibodies. This includes phospho-specific and total antibodies. Now I can already hear the clicking, comments, and hashtags, asking about "what about the phosphatases in milk?" Well, there are some papers out there that discourage you from using milk for phospho signal.
Let me address that by saying that in all of our in-house tests, we don't see any of these issues. CST scientists run so many westerns that we end up making milk at least once a day, sometimes multiple times a day. Now, if your milk buffer goes unused for a week, two weeks, even longer, that increases your chances of phosphatases affecting your signal. But again, we don't see any of these effects, because we use milk buffer fresh on a daily basis.
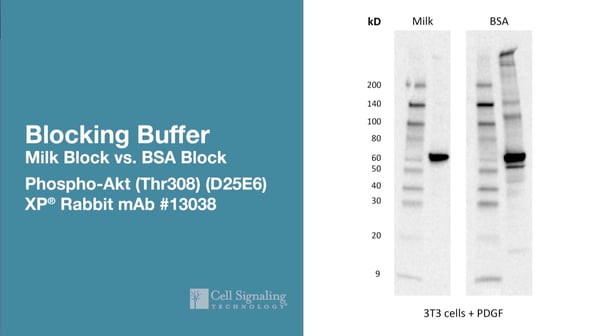
These are images of product Phospho-Akt (Thr308) (D25E6) XP® Rabbit mAb #13038 tested on lysates made from 3T3 cells treated with PDGF. The only difference between the blots is that one membrane is blocked in milk, and the other membrane is blocked in BSA. Clearly, the membrane blocked in BSA has a much higher background. Yet, phospho signal is still strong and clean with the membrane blocked in milk.
So after the membrane has been blocked, please refer to the product's specific datasheet for the recommended antibody collusion buffer. It's either gonna be milk or BSA, depending on the specific antibody. I hope this has been helpful. For full application-specific protocols, they're available on cellsignal.com on a specific product page. If you have any other questions, please feel free to contact any of the scientists at CST at cellsignal.com/support.
For more CST Tech Tip videos, please subscribe to our YouTube page. Good luck with your experiments, and we'll see you next time! Thanks.
Cell Signaling Technology, CST, and XP are trademarks of Cell Signaling Technology, Inc.