The evolution of solid tumor cancer treatment has historically centered on a tripartite approach that includes surgery, chemotherapy, or radiation therapy, and other tumor-specific treatments. More recently, the emergence of immunotherapy has signaled a potential paradigm shift in how tumors of these types are treated.
Immunotherapies such as antibody blockade of T cell co-inhibitory receptors programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), also known as immune checkpoint blockade, strive to unleash the patient's own immune system in order to eradicate the tumor. Unfortunately, many patients do not reap the benefits of these treatments, as the effectiveness of immunotherapy treatments is subject to factors such as a failure of tumor T cell infiltration and an immunosuppressive tumor microenvironment. These “cold tumors” present a barrier to more widespread success of immunotherapy for cancer treatment.
 |
Explore the recombinant monoclonal TIGIT antibody from CST, which is validated for use in immunohistochemistry, immunofluorescence, and WB: • TIGIT (E5Y1W) XP® Rabbit mAb #99567 |
|
Given the limited but remarkable success of immune checkpoint blockade, recent research efforts have been focused on identifying new targets for improving immunotherapy effectiveness in the immunosuppressive tumor microenvironment. One such target is T cell immunoreceptor with Ig and ITIM domains (TIGIT).
What is TIGIT?
TIGIT is a co-inhibitory receptor expressed by regulatory T cells, activated CD8+ and CD4+ T cells, and natural killer (NK) cells1. TIGIT has been shown to alter the production of IL-12 and IL-10 through binding competition for CD155 on dendritic cells (DCs), and can also directly inhibit the function of T cells and NK cells, both impeding T cell proliferation and NK cell cytotoxicity and IFN-γ production.2 TIGIT is also believed to hinder CD226 activation, with the expression balance between the two required for proper regulation of CD4+ T cell T-bet expression and IFN-γ production.2 TIGIT has multiple mechanisms of immune inhibition, emphasizing its importance as a research target for improving immunotherapies.
TIGIT and T-Cell Exhaustion
An additional obstacle to effective immunotherapy is T-cell exhaustion. Exhausted T cells do not respond well to immunotherapy, and TIGIT likely plays a role in this as well. TIGIT is expressed by terminally exhausted CD8+ T cell subsets in tumors, alongside other co-inhibitory receptors such as PD-1, CTLA-4, T cell immunoglobulin, and mucin domain-containing molecule-3 (TIM-3), and lymphocyte activation gene 3 (LAG-3). Coexpression of TIGIT and other co-inhibitory receptors can abrogate proliferation and cytokine production of tumor antigen-specific CD8+ T cells.3,4
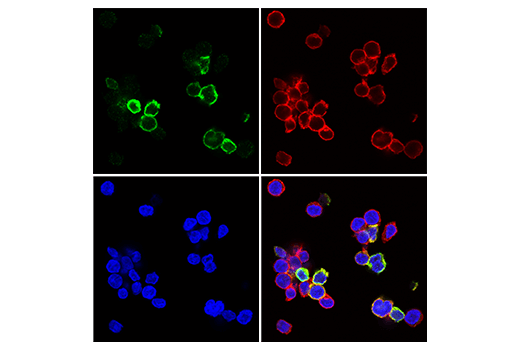 Immunofluorescent analysis of human CD8+ T cells using TIGIT (E5Y1W) XP® Rabbit mAb #99567 (green) and CD8α (RPA-T8) Mouse mAb (FITC Conjugate) #55397 (red pseudocolor). Samples were mounted in ProLong® Gold Antifade Reagent with DAPI #8961 (blue). CD8+ T cells were purified from human blood and stimulated for 7 days using beads coated with CD3 and CD28 antibodies in the presence of Human Interleukin-2 (hIL-2) #8907 (20 ng/mL).
Immunofluorescent analysis of human CD8+ T cells using TIGIT (E5Y1W) XP® Rabbit mAb #99567 (green) and CD8α (RPA-T8) Mouse mAb (FITC Conjugate) #55397 (red pseudocolor). Samples were mounted in ProLong® Gold Antifade Reagent with DAPI #8961 (blue). CD8+ T cells were purified from human blood and stimulated for 7 days using beads coated with CD3 and CD28 antibodies in the presence of Human Interleukin-2 (hIL-2) #8907 (20 ng/mL).
Accumulation of CD8+ exhausted T cells and/or CD4+ T regulatory cells expressing high levels of TIGIT may act as biomarkers of tumors that are unresponsive to immunotherapy.2,5 Dual TIGIT and PD-1 blockade has been shown to improve outcomes in several tumor types, through improved NK cell antitumor activity and CD8+ T cell proliferation and function.6
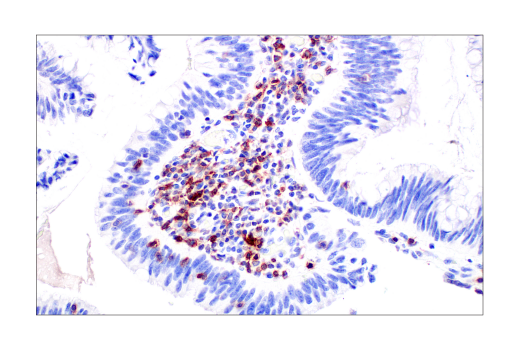 TIGIT positive immunohistochemical analysis of paraffin-embedded human colon adenocarcinoma using TIGIT (E5Y1W) XP® Rabbit mAb #99567.
TIGIT positive immunohistochemical analysis of paraffin-embedded human colon adenocarcinoma using TIGIT (E5Y1W) XP® Rabbit mAb #99567.
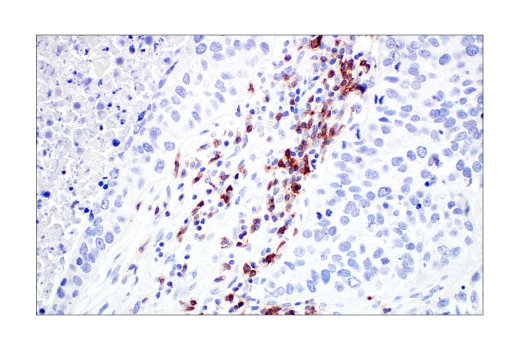 TIGIT positive Immunohistochemical analysis of paraffin-embedded human non-small cell lung carcinoma using TIGIT (E5Y1W) XP® Rabbit mAb #99567.
TIGIT positive Immunohistochemical analysis of paraffin-embedded human non-small cell lung carcinoma using TIGIT (E5Y1W) XP® Rabbit mAb #99567.
TIGIT Antibodies for Immunotherapy Research
Given the potential of targeting TIGIT for immunotherapy research, there is a strong need for robust, validated TIGIT antibodies. Driven to meet that need, CST's TIGIT antibody is fully validated for WB, IF, and most importantly, IHC. This will facilitate reliable and accurate tissue analysis, an especially important factor for studies evaluating the effectiveness of immunotherapy treatments in solid tumors involving or targeting TIGIT.
Pairing this TIGIT antibody with other immune checkpoint antibodies can aid researchers in best understanding, targeting and manipulating these pathways to improve immunotherapy effectiveness and combat T cell exhaustion in solid tumors and more.
References
- Liu, X., Hou, M. & Liu, Y. TIGIT, A Novel Therapeutic Target for Tumor Immunotherapy. Immunological Investigations 46, 172–182 (2017). PMID: 27819527
- Chauvin, J.-M. & Zarour, H. M. TIGIT in cancer immunotherapy. J Immunother Cancer 8, (2020). PMID: 32900861
- Johnston, R. J. et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 26, 923–937 (2014). PMID: 25465800
- Chauvin, J.-M. et al. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest 125, 2046–2058 (2015). PMID: 25866972
- Yang, Z.-Z. et al. TIGIT Expression Is Associated with T-cell Suppression and Exhaustion and Predicts Clinical Outcome and Anti-PD-1 Response in Follicular Lymphoma. Clin Cancer Res 26, 5217–5231 (2020). PMID: 32631956
- Zhang, Q. et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nature Immunology 19, 723–732 (2018). PMID: 29915296







