Previously considered distinct disciplines, the fields of immunology and metabolism are converging in a groundbreaking realization that metabolic processes intricately regulate immune cell function. Known as immunometabolism, the field explores the intricate interplay between metabolism and immune function and helps unravel the complex web of interactions that shape how our body responds to pathogens, diseases, and therapies. Current studies seek to understand how metabolic processes influence the behavior of immune cells such as their development, activation, and function, ultimately hoping to identify new therapeutic targets.
In the tumor microenvironment (TME), the interaction between metabolism and immune function is particularly significant as it directly impacts tumor progression, evasion of immune surveillance, and response to immunotherapy. This post gives a brief overview of our current understanding of the landscape of glucose metabolism in tumor cells and immune cells, context that is useful for understanding how a metabolic checkpoint between glycolysis and the TCA cycle regulates immune response and inflammation.
At the end of this post, you’ll also find a list of the relevant CST products for studying immunometabolism in the TME.
<Jump to the immunometabolism target product list>
Immunometabolism in the Tumor Microenvironment
Cancer mass consists of cancer cells and various non-cancer cells, including different types of immune cells. These heterogeneous types of cells, along with blood vessels, signaling molecules, metabolites, and the extracellular matrix, constitute a complex ecosystem known as the TME.
Webinar: Key Signaling Pathways in Cancer: Emerging Trends in Immunometabolism
Within the TME, immune cells play a critical role in regulating cancer progression. Avoiding immune destruction and tumor-promoting inflammation are highlighted as two hallmarks of cancer, emphasizing the roles of immune cells in the TME. Simultaneously, metabolism in immune cells is critical for immune cell differentiation, phenotype conversions, and effector functions, and as such, studies of intracellular metabolic changes in immune cells are an integral part of the field of immunometabolism.
Moreover, cell-extrinsic factors in the TME, such as tissue vascularization, nutrient and oxygen availability, and crosstalk between immune cells and other cell types further influence metabolic activity in immune cells. Notably, the location of immune cells within tissue has a crucial impact on their metabolic behavior.
Glucose Metabolism and a Metabolic Checkpoint
Glucose is an essential component of glycolysis, a ten-step metabolic pathway used by cells to convert glucose into energy. After the cellular uptake of glucose by glucose transporter 1 (Glut1, SLC2A1), glucose is broken down in the cytosol via glycolysis to generate pyruvate, ATP, and NADH. Subsequently, in cells with sufficient oxygen, pyruvate translocates to the mitochondrial matrix, where it is converted to acetyl-CoA by the pyruvate dehydrogenase complex (PDC). Acetyl-CoA then enters the tricarboxylic acid (TCA) cycle in the mitochondrial matrix, where it generates NADH and FADH2. NADH and FADH2 are oxidized in the electron transport chain in the mitochondrial inner membrane to generate ATP.
Blog: What is glycolysis and what is its role in metabolism?
However, when oxygen is scarce, pyruvate dehydrogenase (PDH) activity is inhibited, causing pyruvate from glycolysis to be diverted to lactate production via lactate dehydrogenase (LDHA). PDH activity is regulated via phosphorylation by pyruvate dehydrogenase kinase (PDHK). PDH activity affects the relative activity of glycolysis and the TCA cycle. Therefore, PDH is a regulated metabolic checkpoint that influences cellular energy production and metabolic adaptation in response to varying oxygen levels.
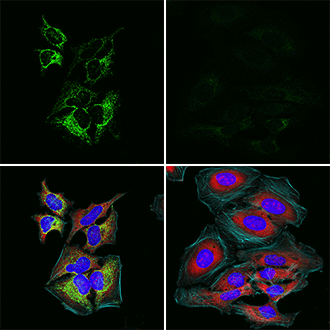
In the above IF analysis, HeLa cells were mock-treated (left), or treated with 5 mM sodium dichloroacetate, a pyruvate dehydrogenase kinase (PDHK) inhibitor, for 16 hours to reduce phosphorylation of Ser293 (right) using Phospho-Pyruvate Dehydrogenase α1 (Ser293) (E4V9L) Rabbit mAb #37115 (green) and Phospho-S6 Ribosomal Protein (Ser235/236) (E2R1O) Mouse mAb #62016 (red).
In cancer cells, the redirection of pyruvate to lactate production in oxygen-rich environments is referred to as aerobic glycolysis or the Warburg effect. A hallmark of proliferative metabolism, the phenomenon is a metabolic reprogramming used by cancer cells that allows them to employ glycolysis for energy production, even when sufficient oxygen is available. The effect is believed to help enable the increased proliferation and enhanced survival rates of many types of cancer cells.
Resource: Warburg Effect Pathway Diagram
The Metabolic Checkpoint's Role in Immunity and Cancer
As described, the metabolic checkpoint between glycolysis and the TCA cycle plays a pivotal role in regulating cellular function, particularly in adaptive immune responses and cancer progression.
Below we describe how this metabolic checkpoint influences different immune cell types and their functions, as well as its impact on cancer cell metabolism.
- Differential regulation of the activation of M1-like macrophages and M2-like macrophages in innate immunity: Phosphorylation of pyruvate dehydrogenase (PDH) by pyruvate dehydrogenase kinase (PDHK) is required for M1-like macrophage polarization. In the polarization process, expressions of PDHK1, PDHK3, and PDHK4 are elevated, leading to the phosphorylation of PDH and inhibition of its activity. This metabolic change pivots cells from oxidative phosphorylation in mitochondria to glycolysis in the cytosol. Knockdown of PDHK1 or deletion of both PDHK2 and PDHK4 reduces glycolytic activity and suppresses M1 polarization while increasing mitochondrial respiration and increasing polarization toward M2 activity.1-3
- Differential regulation of the proliferation, differentiation, and survival of CD4+ effector T cells (Teff) and regulatory T cells (Treg) in adaptive immunity: Activated CD4+ T cells differentiate into effector T cells (Teff) and regulatory T cells (Treg). Studies of CD4+ T cell populations in murine models show that PDHK1 is expressed in higher levels in the effector Th17 cells than in regulatory T cells. Inhibition of PDHK1 activity or downregulation of PDHK1 expression decreases glycolysis and reduces the effector Th17 cell proliferation, differentiation, and survival. On the other hand, inhibition of PDHK1 enhances the TCA cycle and oxidative phosphorylation in mitochondria and promotes differentiation of regulatory T cells. These observations indicate that the effector Th17 cells and regulatory T cells differentially require glycolysis and mitochondrial respiration, respectively, to proliferate, differentiate, and survive.4
Pro-inflammatory immune cells such as M1-like macrophages and effector Th17 cells take up more glucose and enhance aerobic glycolysis. In contrast, anti-inflammatory immune cells such as M2-like macrophages and regulatory T cells use more oxidative phosphorylation.5
- Aerobic glycolysis in cancer cells: Cancer cells take up a large amount of glucose and convert it to pyruvate through glycolysis, followed by lactate fermentation, even when there is plenty of oxygen (aerobic glycolysis, the Warburg effect). Aerobic glycolysis generates increased levels of biosynthetic intermediates, energy (ATP), and reducing equivalent (NADPH) to support the biosynthesis of macromolecules for cancer cell proliferation. In cancer cells, the expression and activity of PDHK1 are regulated. For example, PDHK1 expression is upregulated by HIF-1α and c-Myc. In addition, PDHK1 was shown to be phosphorylated at Tyr243 by a variety of oncogenic tyrosine kinases in some cancer cells, leading to increased enzymatic activity. Enhanced PDHK1 activity promotes a metabolic shift toward glycolysis.6
The graphics below detail the macrophage and T-cell markers that can be used to study immunometabolism in human and murine immune cells.
 View the full Human Immune Cell Marker Guide to explore markers for additional cell types in the human immune system.
View the full Human Immune Cell Marker Guide to explore markers for additional cell types in the human immune system.
View the full Mouse Immune Cell Marker Guide to explore markers for additional cell types in the murine immune system.
Why it Matters…
Cellular metabolism has a key role in regulating the function of immune cells. Research in the emerging field of immunometabolism is expected to offer new perspectives on how metabolism influences the immune system.
CST Antibody Products for Immunometabolism Research
T-Cell Markers |
|
| Th17 | Treg |
| IL-17A (D1X7L) Rabbit mAb #13838 | FoxP3 (D6O8C) Rabbit mAb #12632 |
| IL-17RA (D1Y4C) Rabbit mAb #12661 | IL-2Rα/CD25 (D6K5F) Rabbit mAb #13517 |
| IL-17F (D3M4D) Rabbit mAb #13186 | |
Select References:
- Jeon JH, Thoudam T, Choi EJ, Kim MJ, Harris RA, Lee IK. Loss of metabolic flexibility as a result of overexpression of pyruvate dehydrogenase kinases in muscle, liver and the immune system: Therapeutic targets in metabolic diseases. J Diabetes Investig. 2021;12(1):21-31. doi:10.1111/jdi.13345
- Tan Z, Xie N, Cui H, et al. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol. 2015;194(12):6082-6089. doi:10.4049/jimmunol.1402469
- Min BK, Park S, Kang HJ, et al. Pyruvate Dehydrogenase Kinase Is a Metabolic Checkpoint for Polarization of Macrophages to the M1 Phenotype. Front Immunol. 2019;10:944. Published 2019 May 7. doi:10.3389/fimmu.2019.00944
- Gerriets VA, Kishton RJ, Nichols AG, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125(1):194-207. doi:10.1172/JCI76012
- Pålsson-McDermott EM, O'Neill LAJ. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020;30(4):300-314. doi:10.1038/s41422-020-0291-z
- Hitosugi T, Fan J, Chung TW, et al. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44(6):864-877. doi:10.1016/j.molcel.2011.10.015
Alexandra Foley, Scientific Marketing Writer and CST Blog Manager, contributed to writing this post. 24-HMC-64201
24-HMC-64201






