For antigenic targets where expression of the protein is very low or unknown, the use of recombinant proteins or exogenous expression in a surrogate cell line may be necessary for antibody validation. Although endogenous systems are preferred for their closer representation of in vivo conditions, the heterologous strategy offers several advantages.
Testing for Antibody Cross-Reactivity
A heterologous strategy can be used to verify the cross-reactivity of an antibody with protein isoforms or conserved family members, providing useful information regarding the antibody’s potential for off-target binding based on antigen homology. A heterologous strategy can also be used to test the sensitivity of an antibody by titrating the target protein by expression or dilution.
Additional uses of heterologous strategy include validating and optimizing the ability of an antibody to work in immunoprecipitation (IP) and generating a standard positive control for western blot (WB) applications. Moreover, by providing the option to express various, subtly different forms of a protein, heterologous strategy allows for verification of antibody specificity against site-specific mutants.
Using Recombinant Proteins to Verify Antibody Specificity
A common use of recombinant proteins is to verify the specificity of an antibody for one or more members of a protein family. To illustrate this, Figure 1 shows a WB analysis of nine different recombinant protein kinase C (PKC) isoforms to confirm the specificity of the PKCδ (D10E2) Rabbit mAb. In this instance, antibody binding is isoform-specific, with no off-target binding observed for the other recombinant isoforms tested.
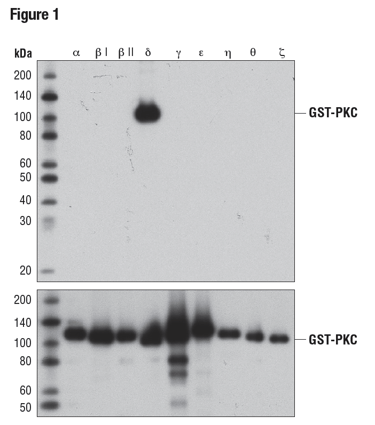
Figure 1: WB analysis of bacterially expressed, GST-tagged, purified PKC isoforms, using PKCδ (D10E2) (upper) or GST (91G1) (lower), demonstrating specificity for PKCδ.
In a slightly different approach, Figure 2 demonstrates the pan-reactivity of a 14-3-3 (pan) antibody. The WB data clearly show antibody recognition of all six recombinant isoforms tested, providing supporting evidence for isoform reactivity. Importantly, the inclusion of a loading control shows differences in band intensity to be due to protein load rather than a result of varying antibody affinity for different isoforms.
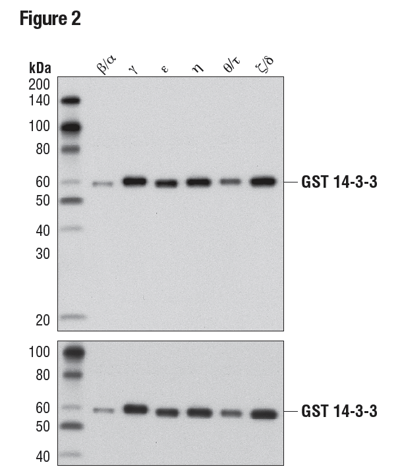 Figure 2: WB analysis of purified, recombinant, GST-tagged 14-3-3 isoforms using 14-3-3 (pan) (upper) or GST (91G1) (lower), demonstrating isoform cross-reactivity.
Figure 2: WB analysis of purified, recombinant, GST-tagged 14-3-3 isoforms using 14-3-3 (pan) (upper) or GST (91G1) (lower), demonstrating isoform cross-reactivity.
A key point to note here is that, while recombinant protein or peptide arrays can be used to determine antibody specificity and cross-reactivity, results from these types of strategies can be misleading due to the artificial nature of these assays. Moreover, several key features of these systems currently lack standardization, highlighting once again the need to back any heterologous strategy with the other hallmarks outlined in this handbook.
Heterologous Expression to Verify Antibody Specificity and Cross-Reactivity
DNA plasmids designed to express the antigenic target(s) of interest in a heterologous system are a popular alternative to using recombinant proteins. Again, this strategy is commonly used to verify antibody specificity and determine cross-reactivity with isoforms, homologs, and orthologs. Proteins expressed in this way are readily analyzed by established techniques, such as western blot or immunocytochemical staining.
Heterologous expression is a required step in validating antibodies against epitope tags, proteins that are not endogenously expressed in mammalian cells, or proteins that are expressed under only a limited set of conditions or in rare cell populations. Since these targets cannot be analyzed in endogenous systems, heterologous expression is essential to verify antibody specificity.
Figure 3 shows a WB analysis of a MAGE-A3 antibody using lysates prepared from 293T cells following transfection with constructs expressing various Myc/DDK-tagged MAGE isoforms. MAGE-A3 is a nuclear protein frequently expressed in various tumor cells and is not endogenously expressed by 293T. These data illustrate antibody specificity for MAGE-A3, with a DYKDDDK antibody used to demonstrate successful transfection and a β-actin antibody employed as a loading control.
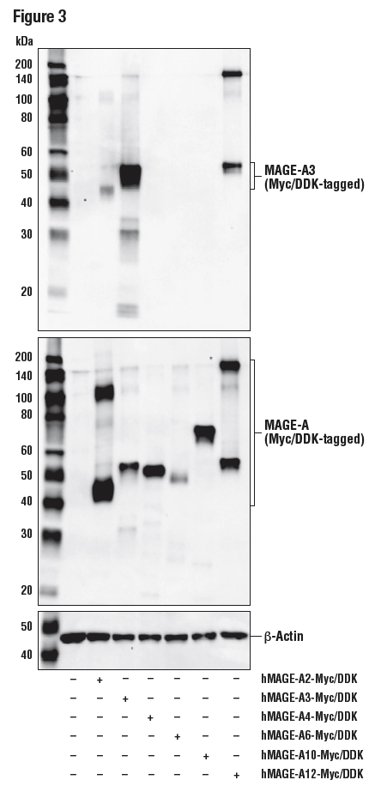
Figure 3: WB analysis of extracts from 293T cells, mock transfected (-) or transfected (+) with constructs expressing Myc/DDK-tagged full-length human MAGE-A2 (hMAGEA2-Myc/DDK), Myc/DDK-tagged full-length human MAGE-A3 (hMAGE-A3-Myc/DDK), Myc/DDK-tagged full-length human MAGE-A4 (hMAGE-A4-Myc/DDK), Myc/DDK-tagged full-length human MAGE-A6 (hMAGE-A6-Myc/DDK), Myc/DDK-tagged full-length human MAGE-A10 (hMAGE-A10-Myc/DDK), and Myc/DDK-tagged full-length human MAGE-A12 (hMAGE-A12-Myc/DDK), using MAGE-A3 (E9S4X) (upper), DYKDDDK Tag Antibody (middle), and β-Actin (D6A8) (lower).
In Figure 4, data illustrating the validation of an antibody to the non-natively expressed protein Cas9 are shown in a model consistent with its intended experimental use. Here, Myc-tagged Cas9 was transfected into 293T cells and detected using the Cas9 (S. pyogenes) (E7M1H) rabbit mAb to perform immunocytochemical staining. The Myc-Tag (9B11) mouse mAb was used to confirm expression of the cDNA.
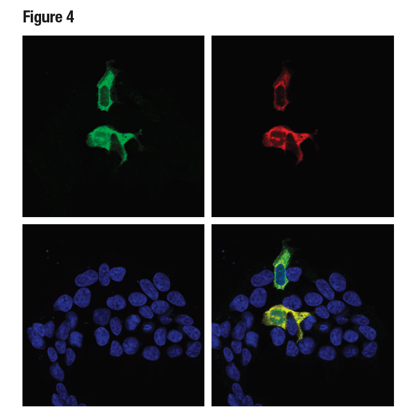 Figure 4: Confocal ICC analysis of 293T cells transiently transfected with a Myc-tagged Cas9 (S. pyogenes) construct, using Cas9 (S. pyogenes) (E7M1H) (green) and Myc-Tag (9B11) (red). Colocalization of green and red signals appear yellow in the composite image (bottom-right). Samples were mounted in ProLong® Gold Antifade Reagent with DAPI (blue).
Figure 4: Confocal ICC analysis of 293T cells transiently transfected with a Myc-tagged Cas9 (S. pyogenes) construct, using Cas9 (S. pyogenes) (E7M1H) (green) and Myc-Tag (9B11) (red). Colocalization of green and red signals appear yellow in the composite image (bottom-right). Samples were mounted in ProLong® Gold Antifade Reagent with DAPI (blue).
Detection of Site-Specific Mutants
Because a heterologous strategy allows for the expression of an almost infinite number of constructs, it is not limited to expressing wild-type proteins. Confirmation of the specificity of a site-specific antibody can be verified using site-specific mutants of the protein expressed exogenously/recombinantly in bacterial or mammalian cells.
Illustrating this concept, Figure 5 demonstrates specificity of the phospho-p90RSK (Thr359) (D1E9) rabbit mAb for the Thr359-phosphorylated form of the p90RSK protein. In this instance, the antibody was used to probe lysates prepared from cells expressing the wild-type protein, a Thr359 to Ala (T359A) mutant, a Ser362 to Ala (S362A) mutant, or a dual mutant (T359A/S362A), in addition to equivalent sites on p90RSK family members RSK2 and RSK3.
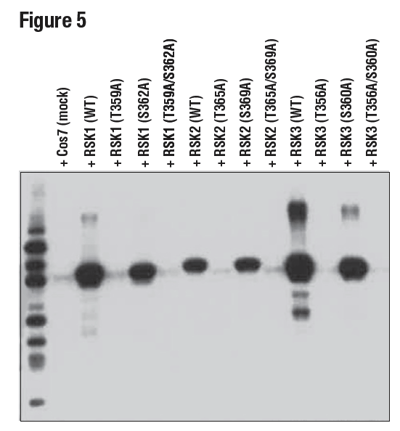 Figure 5: WB analysis of extracts from 293T cells, mock-transfected (mock), or transfected with constructs expressing wild-type (WT) RSK1, RSK2, and RSK3, or the indicated site-specific mutations, using Phospho-p90RSK (Thr359) (D1E9), demonstrating phospho-specific reactivity of the antibody with RSK1 (Thr359), RSK2 (Thr365), and RSK3 (Thr356).
Figure 5: WB analysis of extracts from 293T cells, mock-transfected (mock), or transfected with constructs expressing wild-type (WT) RSK1, RSK2, and RSK3, or the indicated site-specific mutations, using Phospho-p90RSK (Thr359) (D1E9), demonstrating phospho-specific reactivity of the antibody with RSK1 (Thr359), RSK2 (Thr365), and RSK3 (Thr356).
These results highlight two important features of the antibody: specificity for the threonine site and a capacity to equivalently detect the conserved site on other family members. These kinds of experiments are rarely done or are overlooked by most vendors.
Since heterologous strategy differs significantly from the other hallmarks in that it relies on non-native expression of antigenic targets, it is of paramount importance to combine it with other validation strategies to achieve meaningful insight regarding antibody specificity and sensitivity. Used in isolation, heterologous strategy can be misleading, but in combination with the other hallmarks it represents an extremely powerful approach to antibody validation.
Additional Antibody Validation Resources
Read the additional blog posts in our Hallmarks of Antibody Validation series:
- Introduction to the Hallmarks of Antibody Validation
- Hallmarks of Antibody Validation - Binary Strategy
- Hallmarks of Validation - Ranged Strategy
- Hallmarks of Validation - Orthogonal Strategy
- Hallmarks of Validation - Multiple Antibody Strategy
- Hallmarks of Validation - Heterologous Strategy
- Hallmarks of Validation - Complementary Strategies






