Flow cytometry offers the exciting ability to detect and quantify multiple readouts per cell, enabling you to gain multidimensional insight into your system of interest. Combining multiple antibodies conjugated to different fluorescent molecules, or fluorophores, is a common approach. But which fluorophores will stand out from one another and be easy to differentiate? Which will result in highly overlapping fluorescence and uninterpretable data? Learning which fluorophores may be used together can be time-consuming when you are new to flow cytometry, or are working with unfamiliar fluorophores. We have developed a set of easy-to-use resources to help you choose compatible fluorophores quickly.
The tried-and-true method for determining whether two or more fluorophores may be used in combination is to view their excitation and emission spectra on a spectral viewer. Viewing their spectra will enable you to see whether the fluorophores will be excited by the same color of light, and how much overlap their emitted light will have. Less overlap is better, since overlapping light will require compensation to mathematically remove the contribution of light from fluorophores other than the one of interest. When your assay only consists of a few readouts, it is possible to select a panel of fluorophores that requires little-to-no compensation. This will save a lot of setup time. It will also reduce the level of uncertainty in the resulting data.
Compatible Fluorophore Combinations for Small Flow Cytometry Panels
Using a spectral viewer, especially in combination with a panel builder, is very helpful if you are building a large panel of antibodies and need to see overlap for many fluorophores. But what if you have a single fluorophore and just want to know what you can pair it with? Loading all the different spectra can be time-consuming when all you need is a simple yes or no answer to assess your options. To help you find that answer more quickly, we have generated a quick reference table (below) to show you which commonly used fluorophores can be combined with little or no compensation required. This table also shows which fluorophores should not be combined, as they would yield highly overlapping fluorescent signal that could only be differentiated using a spectral flow cytometer (sometimes with great uncertainty).
Two tables are provided, one for each type of light path used in a flow cytometer. Parallel excitation means that each excitation source hits the target independently, and the resulting fluorescent emission is measured independently. Alternatively, in co-linear excitation, the excitation sources all converge on the object together and the resulting emission spectra are quantified together. Thus, two dyes with different excitation sources but highly overlapping emission spectra may be used together on an instrument with parallel excitation, but they could not be differentiated on an instrument with co-linear excitation. Use the table that corresponds with the light path of the flow cytometer you will use.
Flow Cytometry Compatibility Tables for Novice Cytometrists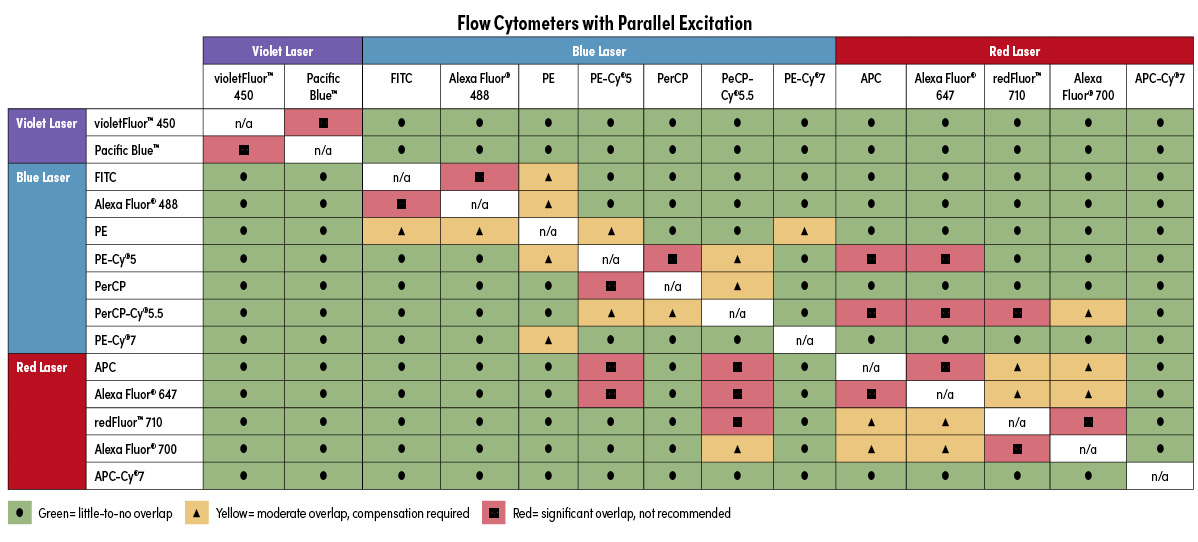

Wondering how to combine multiple antibodies in your assay when they are recommended with different protocols? Check out our Antibody Staining Guide for Flow Cytometry to determine how to best combine antibodies binding extracellular and intracellular targets.
We can also browse the antibody conjugates available on the CST website, including antibody conjugates that are validated for flow cytometry experiments:
We hope this is helpful. If you have any questions about fluorophores or using our antibodies for flow cytometry assays, please reach out to our team.







