Rajan Burt, Samuel Myers, and colleagues from the La Jolla Institute for Immunology, Broad Institute, Cell Signaling Technology, and Yale University combined their expertise to successfully pioneer a new path toward identifying the ubiquitous protein modification O-GlcNAcylation. The results of these efforts are published in the paper Novel Antibodies for the Simple and Efficient Enrichment of Native O-GlcNAc Modified Peptides in the journal Molecular & Cellular Proteomics.
When Specificity Is Simply Sweet
The story is an old one, as the simple sugar or monosaccharide O-GlcNAc was identified almost 40 years ago by Torres and Hart in 1984. O-GlcNAc is one of the most common post-translational modifications (PTM). It’s found everywhere on proteins inside the cell, including the nucleus, cytoplasm, and mitochondria. Not to be confused with the highly complex N-linked sugars present on proteins that face the extracellular milieu, O-GlcNAc is a simple monosaccharide involved in the most complicated biological pathways involving the epigenetic regulation of transcription, stress response, translation, protein degradation, and homeostasis.
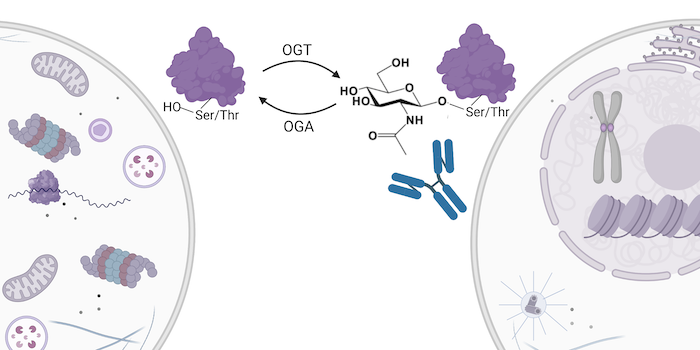
Because it functions as a cellular sensor for nutrient levels, O-GlcNAc serves as an integrator of multiple signaling pathways. Remarkably, O-GlcNAcylation is controlled by only two proteins cell-wide: OGT, which adds the monosaccharide to serine and threonine residues, and OGA, which enzymatically removes the PTM. Newer, more holistic models of signal transduction shy away from merely linear models of activation and inhibition to models that incorporate multiple pathways as well as multiple modes of feedback and cross-talk. This explains, in part, how one PTM can be involved in seemingly diverse diseases like diabetes, neurodegeneration, and cancer in humans.
A Sensitive, Specific Monoclonal Antibody for O-GlcNAc
The key to understanding the role of O-GlcNAc in biology and disease is reliably identifying the PTM. Scientists at Cell Signaling Technology have created a mixture of sensitive, specific rabbit monoclonal antibodies that can immunoprecipitate in one step the O-GlcNAcylated peptides derived from the parent proteins. This new antibody-based tool, coupled with advances in mass spectrometry developed by scientists at the Broad Institute, allow for the identification of over a thousand unique O-GlcNAcylated sites in one enrichment step.
![PTMScan® O-GlcNAc [GlcNAc-S/T] Motif Kit](https://media.cellsignal.com/product/image/95220_fig02___20210423082456.png) Motif analysis using tryptic peptides enriched and identified by PTMScan® O-GlcNAc [GlcNAc-S/T] Motif Kit. Ten milligrams of HeLa cells treated with 10 μM Thiamet-G (TMG) for 6 hours were digested with trypsin and immunoprecipitated with PTMScan® O-GlcNAc [GlcNAc-S/T] Immunoaffinity Beads. Orbitrap Fusion Lumos mass spectrometer analysis identified a total of 1,235 non-redundant sites.
Motif analysis using tryptic peptides enriched and identified by PTMScan® O-GlcNAc [GlcNAc-S/T] Motif Kit. Ten milligrams of HeLa cells treated with 10 μM Thiamet-G (TMG) for 6 hours were digested with trypsin and immunoprecipitated with PTMScan® O-GlcNAc [GlcNAc-S/T] Immunoaffinity Beads. Orbitrap Fusion Lumos mass spectrometer analysis identified a total of 1,235 non-redundant sites.
Furthermore, the sensitivity and specificity of the O-GlcNAc antibody was demonstrated by a strong preference for O-GlcNAc as compared to its epimer O-GalNAc. The technique may be readily applied to native O-GlcNAc modified peptides, allowing investigators to explore O-GlcNAc questions in vivo.
So, without complicated and time-consuming chemical derivatization, using 10-fold less sample than previous experiments, and with significantly less mass spectrometer time, this study demonstrates the synergy of highly specific reagents and innovative mass spectrometry in creating a pathway for discovery.
21-ETC-55250






