Post-translational modifications (PTMs) are a critical regulatory mechanism that allows cells to tightly control protein function. They are reversible, covalent modifications that alter the protein state, such as protein folding, protein-protein interactions, protein-DNA interactions, protein lifespan, protein location, and receptor activation. These behaviors, in turn, control biological processes like enzyme activity, cell cycle, apoptosis, and gene expression.
Common Post-Translational Modifications
Some common post-translation modifications include phosphorylation, acetylation, and methylation. A protein can be modified on several different amino acids with different modifications, where different PTM combinations result in different protein behaviors. For example, p53 is modified by as many as 50 individual post-translational modifications.
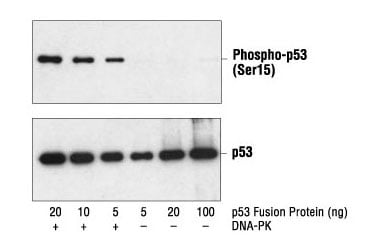 Western blot analysis of a p53 fusion protein, untreated or phosphorylated by DNA-PK, using Phospho-p53 (Ser15) Antibody #9284 (upper) and p53 Antibody #9282 (lower).
Western blot analysis of a p53 fusion protein, untreated or phosphorylated by DNA-PK, using Phospho-p53 (Ser15) Antibody #9284 (upper) and p53 Antibody #9282 (lower).
Phosphorylation on p53 at Ser15 and Ser20 can block interactions with E3 ubiquitin-protein ligase MDM2 to promote accumulation and activation of p53 in response to DNA damage; phosphorylation on Ser46 plays a role in apoptosis induction; acetylation on Lys164 induces cell cycle arrest; and acetylation on Lys373 results in hyperphosphorylation of p53 N-terminal residues that in turn enhances the DNA binding ability to promoters for proapoptotic genes.
PTMs on other proteins like histones are similarly complex. For example, acetylation of histone 3 on Lys9 has a dominant role in histone disposition and chromatin assembly while phosphorylation on Ser10, Ser28, and Thr 11 is tightly correlated with chromosome condensation during mitosis and meiosis. Histone 3 methylation, in turn, is primarily associated with transcriptional activation; however, there are scenarios where histone 3 methylation is associated with repression. And that’s just a snapshot of the complexity involved with protein PTMs.
%20(D7N8E)%20Rabbit%20mAb%20%2353348.jpg?width=300&height=400&name=Phospho-Histone%20H3%20(Ser10)%20(D7N8E)%20Rabbit%20mAb%20%2353348.jpg) Western blot analysis of extracts from HeLa cells, either untreated or treated with Nocodazole #2190 (100 ng/ml, 16 hr), using Phospho-Histone H3 (Ser10) (D7N8E) XP® Rabbit mAb #53348 (upper) or Histone H3 (D1H2) Rabbit mAb #4499 (lower). Phospho-specificity of the antibody is shown by further treatment of the lysate with λ phosphatase.
Western blot analysis of extracts from HeLa cells, either untreated or treated with Nocodazole #2190 (100 ng/ml, 16 hr), using Phospho-Histone H3 (Ser10) (D7N8E) XP® Rabbit mAb #53348 (upper) or Histone H3 (D1H2) Rabbit mAb #4499 (lower). Phospho-specificity of the antibody is shown by further treatment of the lysate with λ phosphatase.
Disruptions to the mechanisms that regulate protein PTM states have severe consequences and are found in a wide variety of diseases like cancer, cardiovascular disease, and neurodegenerative diseases. However, we have just started to scratch the surface when it comes to understanding the many different ways proteins can be modified and the implications of those modifications.
Using Western Blotting for PTM Analysis
So how do you monitor the PTM state of proteins in your sample? Western blots are especially useful since you can use highly specific antibodies to asses the total protein against the modification of interest on a particular residue.
Don’t have time to find the ideal antibody pair to use in your WB? Cell Signaling Technology (CST) has identified modification-specific duet pairs for assessing protein activation state. Cells can be treated with a modulator or cultured under specific conditions to induce a specific cell state. The specific PTM antibody can then determine the amount of modified protein present compared to the untreated control, and the amount of modified protein present in comparison to the total amount of protein. This can be extremely informative, but only if your PTM-specific antibodies do not cross-react with other PTMs on the same protein. Additionally, the PTM-specific antibodies need to be sensitive enough to detect even low levels of modified protein.
%20Antibody%20Duet%20%2326168.jpeg?width=520&height=350&name=PhosphoPlus%20GCN2%20(Thr899)%20Antibody%20Duet%20%2326168.jpeg) PhosphoPlus GCN2 (Thr899) Antibody Duet #26168: Western blot analysis of extracts from HT-1376 cells, untreated (-) or treated with UV (50 mJ/cm2, 30 min recovery; +), using Phospho-GCN2 (Thr899) (E1V9M) Rabbit mAb (upper), GCN2 (E9H6C) Rabbit mAb #40457 (middle), or β-Actin (D6A8) Rabbit mAb #8457 (lower).
PhosphoPlus GCN2 (Thr899) Antibody Duet #26168: Western blot analysis of extracts from HT-1376 cells, untreated (-) or treated with UV (50 mJ/cm2, 30 min recovery; +), using Phospho-GCN2 (Thr899) (E1V9M) Rabbit mAb (upper), GCN2 (E9H6C) Rabbit mAb #40457 (middle), or β-Actin (D6A8) Rabbit mAb #8457 (lower).
Western blots are also extremely flexible, so you’ll have several options when designing your experiment.
- Want to multiplex to save on time or sample? Use primary and fluorescently conjugated secondary antibody pairs raised in different species. You could also strip your membrane and re-probe if you want to re-analyze your membrane.
- Looking to amplify your signal? One option is to use a biotinylated primary antibody, a biotinylated secondary antibody, and streptavidin-HRP to localize more peroxidase molecules to where your protein migrates.
- Need to immunoprecipitate your protein of interest? Use primary antibodies conjugated to Sepharose Beads. Or immunoprecipitate your protein of interest with your favorite antibody and Protein A/G agarose or magnetic beads.
You even have a lot of options when it comes to selecting which chemiluminescence substrate to use in your western blot depending on whether you want to maximize signal or signal-to-noise.
Validated Western Blot Antibodies for PTM Analysis
CST is a company of scientists like you, so we know what you’re looking for in your western blot reagents. Plus, all of our antibodies are validated for specific applications and are specific, sensitive, and reproducible—so you can focus on your research!
Additional Resources:
Check out our Western Blot Resource Center where you'll find WB protocols, troubleshooting tips, application notes and more. Below are a few topics we think you'll find useful:
- Western Blot Experimental Design Tips, which includes considerations for fluorescent western blotting.
- Control Treatments by Target, which provides information about treatments that can be used as a positive control for our activation-state-specific antibodies.
- Download our comprehensive Guide to Successful Western Blotting for additional techniques for generating better WB data.