YAP and its paralog TAZ (WWTR1), often referred to collectively as YAP/TAZ, are transcriptional co-activators that play important roles in regulating tissue growth and organ size. YAP and TAZ function—largely independently and with differing expression patterns1—as key signaling molecules within what is often referred to as the Hippo signaling pathway. Widely conserved across evolution, this kinase pathway responds to multiple cellular inputs, including mechanical stress, metabolic signaling, and cell-cell contact, and exhibits significant crosstalk with many classic receptor-mediated signaling pathways, including ERK/MAPK, Wnt/β-catenin, Notch, and TGF-β.
Suppression of the Hippo kinase pathway (the “OFF-state”) leads to phosphorylation state-dependent activation of YAP and TAZ proteins; active YAP or TAZ proteins translocate to the nucleus where they physically interact with Transcriptional Enhanced Associate Domain (TEAD) transcription factors to regulate the expression of genes that control cell proliferation and apoptosis. Aberrant activation of the YAP/TAZ-TEAD axis is observed in several tumor types; more recently, it has been identified as an oncogenic resistance mechanism following treatment with many traditional small-molecule therapies. Abnormal activation of the YAP/TAZ-TEAD signaling axis ultimately manifests as altered gene expression patterns that promote oncogenesis.
 |
Hippo signaling is an evolutionarily conserved pathway that controls organ size by regulating cell proliferation, apoptosis, and stem cell self-renewal. Dysregulation of the Hippo pathway contributes to cancer development. Explore the interactive Hippo Signaling Pathway diagram, along with associated CST products. |
Structural analyses of YAP, TAZ, and TEAD proteins initially led to this pathway being labeled as undruggable using traditional small-molecule approaches. However, it is now being pursued aggressively by numerous groups using strategies that depart from the traditional small-molecule therapeutic approach.
Recently, I attended The American Association for Cancer Research (AACR 2023) annual meeting, where I witnessed exciting breakthroughs by multiple groups targeting the YAP/TAZ-TEAD signaling axis. Fundamental to most of these approaches was the use of small molecules designed to disrupt protein-protein interactions between YAP/TAZ and TEAD proteins (TEAD1-4), thereby disrupting transcriptional outputs that promote tumorigenesis.
<< Jump to a list of YAP, TAZ & TEAD antibodies at the end of this post >>
Therapeutic Breakthroughs Targeting YAP/TAZ-TEAD
Many findings reported at AACR 2023 focused on targeting a specific cysteine moiety found within all TEAD proteins, palmitoylation of which is required to promote physical interactions between TEAD proteins and YAP or TAZ. Promising Phase 1 clinical trial data were presented by Dr Timothy Yap (MD Anderson Cancer Center) on VT3989, developed by Vivace Therapeutics. VT3989 is one of a host of small molecules designed to block TEAD palmitoylation, consequently interfering with YAP-TEAD interactions required for transcriptional regulation. In the trial, VT3989 was well-tolerated by patients with YAP/TAZ-TEAD-driven malignant mesothelioma, and elicited promising and durable responses. The findings were described as the first clinical proof-of-concept for targeting the YAP/TAZ-TEAD signaling axis using a small molecule designed to target post-translational modifications required for YAP/TAZ-TEAD interaction.
Blog: AACR 2023 Recap: Immunotherapy, CAR-T Cell Therapy Research Dominates
Numerous other groups reported pre-clinical data using a similar approach, including Shen and colleagues from Betta Pharmaceuticals. The group presented data on their covalent, irreversible inhibitor of TEAD palmitoylation, BPI-460372, which was shown to effectively suppress the expression of an engineered TEAD reporter gene, in addition to key YAP/TAZ-TEAD targets genes such as CTGF and CYR61. Similarly, Chen and colleagues from SpringWorks Therapeutics described SW-682, a pan-TEAD inhibitor that proved effective at suppressing TEAD-dependent transcription in multiple cell lines bearing mutations that drive aberrant YAP/TAZ-TEAD signaling.
Two other groups reported data using a similar approach, but with next-generation TEAD inhibitors designed for greater paralog specificity. Florian Muller from Sporos BioDiscovery reported favorable data for SPR1 in multiple cancer cell lines and in vivo (xenograft) tumor models, while also demonstrating a favorable safety profile in preclinical animal models. Likewise, two poster presentations from Ikena Oncology demonstrated promising preclinical data for their tailored-specificity molecule, IK-930. In addition to demonstrating improved anti-tumor activity compared to pan-TEAD inhibitors, they demonstrated that IK-930 showed promise in targeting TEAD-dependent tumor resistance mechanisms that have been reported to arise following treatment with EGFR or MEK inhibitors.
Preclinical data presented by Wolf Wiedemeyer from BridGene Biosciences showed that their TEAD palmitoylation inhibitor, BGI-9004, harbored promise not only as a targeted therapy, but also exhibited properties suggesting its potential suitability as a combination therapy. For example, BGI-9004 was shown to potentiate the effects of KRAS inhibitors in a dose-dependent fashion, suggesting that their YAP/TAZ-TEAD inhibitor may offer promise as a combination therapy in multiple tumor types.
As a CST scientist with experience in YAP/TAZ signaling, I was particularly excited to see the poster presentation by Tobias Schmelzle and colleagues from the Novartis Institutes for Biomedical Research (NIBR) demonstrating the efficacy of their molecule, IAG933, in targeting YAP/TAZ-TEAD protein-protein interactions. They showed that IAG933 was able to specifically interfere with binding between YAP/TAZ and all four TEAD paralogs while displaying favorable pharmacokinetics that suggests suitability for the clinical landscape. Compelling data for the efficacy of IAG933 came from elegant bioassays that utilized CST® monoclonal antibodies developed in collaboration with Dr Giorgio Galli, now a lead scientist at NIBR in Basel. For example, the authors used YAP (D8H1X) XP® Rabbit mAb #14074 to demonstrate that treatment with IAG933 elicited reductions in nuclear YAP protein, but more specifically led to physical eviction of YAP protein from chromatin binding sites, and concomitant reductions in YAP/TAZ-TEAD target gene expression. IAG933 demonstrated efficacy in multiple cancer cell lines and elicited sustained anti-tumor responses in xenograft models, while conforming to a promising safety profile.
Despite initial pessimism for the prospects of targeting YAP/TAZ-TEAD signaling in cancer research, it was clearly evident from AACR 2023 that innovative approaches that deviate from traditional small molecule strategies are demonstrating genuine promise in this area. This is welcome news to many, particularly due to the increasingly recognized importance of this pathway in the development of therapeutic resistance to approved therapies. It was also immensely rewarding to see that immunoassay reagents developed at CST made important contributions to this research progress.
Antibody Targets for Studying the YAP/TAZ-TEAD Signaling Pathway
CST has a variety of highly validated antibody targets for studying the YAP/TAZ-TEAD signaling pathway. All CST antibodies are validated in accordance with the CST Hallmarks of Antibody Validation, six complementary strategies used by our scientists to validate antibody target specificity, maximizing the likelihood of generating reproducible immunoassay data.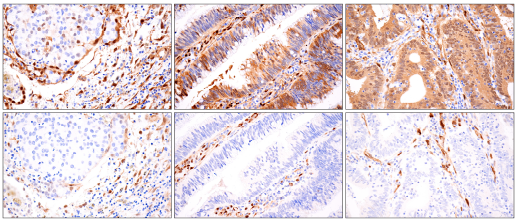
Immunohistochemical analysis of paraffin-embedded human non-small cell lung carcinoma (left), colon carcinoma case 1 (middle), or colon carcinoma case 2 (right), using YAP (D8H1X) XP® Rabbit mAb #14074 (top) or TAZ (E9J5A) XP® Rabbit mAb #72804 (bottom).
Validation of YAP and TAZ monoclonal antibodies in immunohistochemistry is described in detail in the CST scientist-authored publication “YAP vs. TAZ: differences in expression revealed through rigorous validation of target-specific monoclonal antibodies," which received the 2020 Outstanding Publication Award by the Journal of Histotechnology.
Hippo Signaling Antibody Sampler Kits
- Hippo Signaling Antibody Sampler Kit #8579
- Hippo Pathway: Upstream Signaling Antibody Sampler Kit #56612
YAP, TAZ, and TEAD Antibodies from CST
The YAP, TAZ, TEAD, and hippo pathway-related monoclonal antibodies in the table above also come in a variety of conjugates. Don’t see the conjugate you need in our product catalog? We can conjugate it for you.
Learn more about our Custom Antibody Conjugation service.
Select References
- Crosby K, Wood AW, Simendinger J, et al. YAP vs. TAZ: differences in expression revealed through rigorous validation of target-specific monoclonal antibodies. J Histotechnol. 2020;43(4):182-195. doi:10.1080/01478885.2020.1847012
23-CAN-36801






