Understanding when and why cell cycle arrest occurs is critical to many fields of research, including (but not limited to) studies of development, aging, and cancer.
We all know the best tools produce the best results, so make sure you have all your bases covered with this list of the top 10 targets for your senescence research.
Number 1: β-Galactosidase Staining Kit
Senescent cells are known to express β-Galactosidase in a pH-dependent manner, specifically at pH 6.1 Our comprehensive Senescence β-Galactosidase Staining Kit #9860 contains everything you need to detect β-galactosidase activity at pH 6 in cells—or even frozen tissue. Perfect for quickly and easily testing multiple cell populations or tissue samples, the directions are straightforward, and the blue staining is bright and clear.
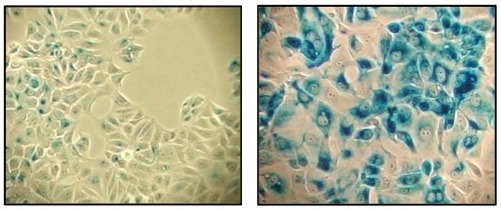
β-Galactosidase staining at pH 6 on MCF-7 cells untreated (left) and senescent MCF-7 cells treated with etoposide #2200 (12.5 μM, 24 hr) and allowed to recover for 4 days (right) using the Senescence β-Galactosidase Staining Kit #9860.
Number 2: p53 and phospho-p53
Tumor protein p53 is so well-studied, it almost needs no introduction. A major player in the DNA Damage Response (DDR) pathways, the p53 tumor suppressor protein is also a critical regulator of the cell cycle. The accumulation of phosphorylated p53 drives the activation of cyclin-dependent kinase inhibitors (CDKIs), ultimately leading to cell cycle arrest.
Confocal Immunofluorescent (IF) analysis of HT-29 cells using p53 (7F5) Rabbit mAb #2527 (green). Actin filaments have been labeled with DY-554 phalloidin (red).
Senescent cell cycle arrest relies heavily on phospho-p53, which accumulates and activates multiple CDKIs. Comparative analysis between p53 and phospho-p53 levels is often a critical step in studying the DDR pathway and senescence.

IF analysis of MCF-7 cells, untreated (left) or etoposide-treated (right), using Phospho-p53 (Ser46) Antibody #2521 (green). Actin filaments have been labeled with DY-554 phalloidin (red).
Number 3: p21
One of the most well-established senescence markers, p21, is a cyclin-dependent kinase inhibitor (CDKI) that is regulated downstream of p53. When associated with CDK2, it acts as an inhibitor of the cell cycle by blocking progression through the G1/S phase.
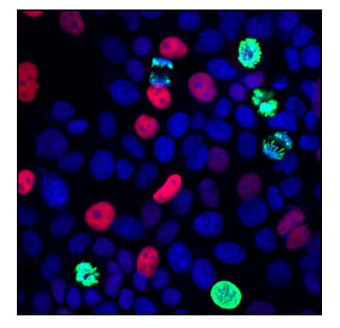
IF analysis of MCF7 cells using p21 Waf1/Cip1 (12D1) Rabbit mAb #2947 (red) and Phospho-Histone H3 (Ser10) (6G3) Mouse mAb #9706 (green). Blue pseudocolor = DRAQ5® #4084 (fluorescent DNA dye).
Number 4: p16
Another common and reliable senescence marker, p16, is a member of the INK4 family of CDKIs. It acts with CDK4 and CDK6 to arrest the cell cycle in the G1 phase.3 The expression of p16 is believed to drive cells into senescence.2
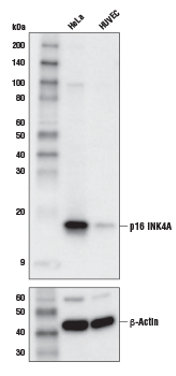
Western blot (WB) analysis of extracts from HeLa and HUVEC cells using p16 INK4A (D3W8G) Rabbit mAb #80772 (upper) or β-Actin (D6A8) Rabbit mAb #8457 (lower).
Number 5: LaminB1
Senescent cells often exhibit morphological changes, making LaminB1 another useful indicator of senescence. A marker for nuclear morphology, LaminB1 expression is lost in senescent human and murine cells.4 Loss of LaminB1, along with increased accumulation of p21 and p16, are all classic hallmarks of senescence.

IF analysis of HT-29 cells using Lamin B1 (119D5-F1) Mouse mAb #68591 (green) and β-Actin (13E5) Rabbit mAb (Alexa Fluor® 647 Conjugate) #8584 (red). Blue pseudocolor = Propidium Iodide (PI)/RNase Staining Solution #4087 (fluorescent DNA dye).
Number 6: Senescence-Associated Secretory Phenotype (SASP)
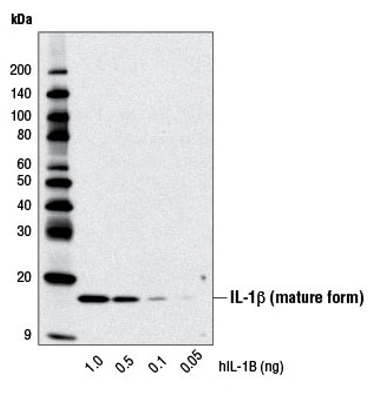
Senescent cells have many common traits, but they are not identical. Each population of senescent cells is characterized by unique levels of cytokines, growth factors, and proteases, which are known as the senescence-associated secretory phenotype (SASP). The SASP Antibody Sampler Kit contains a robust collection of antibodies validated for various applications including western blotting, immunofluorescence, flow cytometry, and immunoprecipitation. The kit will allow you to determine the specific SASP profile of your cell population.
WB analysis of recombinant Human Interleukin-1β (hIL-1β) #8900 using IL-1β (D3U3E) Rabbit mAb.
Number 7: Retinoblastoma protein (Rb) and phospho-Rb
Typically, phosphorylation of the retinoblastoma protein (Rb) is necessary to relieve repression of transcriptional targets and progress the cell cycle. CDKIs, including p21 and p16, inhibit the cell cycle, leading to hyperactivation of Rb and promoting cell cycle arrest and senescence.4
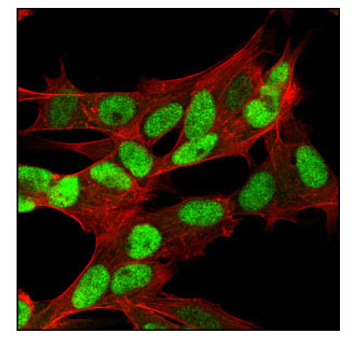
IF analysis of SH-SY5Y cells using RB (4H1) Mouse mAb #9309 (green). Actin filaments have been labeled with Alexa Fluor 555 phalloidin (red).
Since Rb must be phosphorylated to progress the cell cycle, phospho-Rb is absent in senescent cells. Similarly to p53, comparative analysis of Rb and phospho-Rb are crucial when investigating senescence.
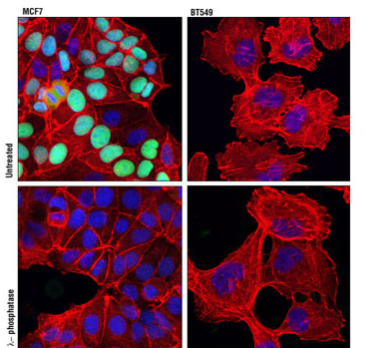
IF analysis of MCF7 (left) and BT-549 (right) cells, untreated (upper) or λ phosphatase-treated (lower) using Phospho-Rb (Ser807/Ser811) (D20B12) XP® Rabbit mAb #8516 (green). Actin filaments were labeled with DY-554 phalloidin (red). Blue pseudocolor = DRAQ® #4084 (fluorescent DNA dye).
Number 8: gamma-H2A.X
gamma-H2A.X, a variant of histone H2A, is a classic marker of the DDR pathway. When DNA is damaged, H2A.X undergoes phosphorylation at Ser139, forming gamma-H2A.X.5 This modification occurs rapidly in robustly in response to DNA damage to mark the sites needed for repair, making it a powerful tool for studying the DDR pathway and senescence.
IF analysis of paraffin-embedded HT-29 cells untreated (left) or UV-treated (right), using Phospho-Histone H2A.X (Ser139) (20E3) Rabbit mAb #9718.
Number 9: 53BP1
53BP1, aptly named p53 binding protein 1, was initially identified as a binding partner for p53, and is suggested to enhance its transcriptional activity.6,7 53BP1 plays an essential role in DNA repair; it is known to be recruited to sites of DNA damage, where its retention is dependent on gamma-H2A.X.8
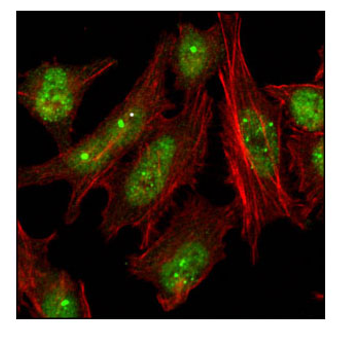
Confocal immunofluorescent analysis of HeLa cells using 53BP1 Antibody #4937 (green). Actin filaments have been labeled with Alexa Fluor® 555 phalloidin (red).
Number 10: Ki-67
Sometimes the best way to detect something is to determine what it’s not doing. Ki-67 is a nuclear protein that is a frequently used marker of proliferating cells. This detection ranges anywhere from the G1 phase through the end of mitosis, but is absent when cells are in G0 resting phase.9 A hallmark of senescent cells is their permanent exit from the cell cycle, and so senescent cells do not express Ki-67.
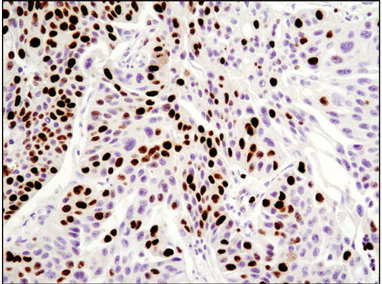
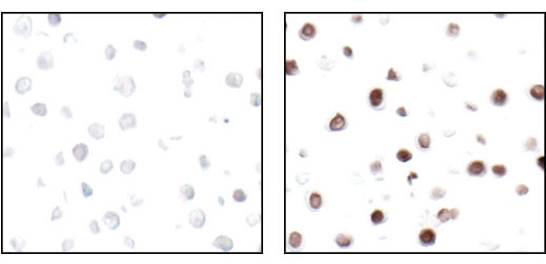
IF analysis of paraffin-embedded human breast carcinoma using Ki-67 (8D5) Mouse mAb #9449.
Antibody Selection for Senescence Research
There are many markers of senescent cells, so why choose just one? We recommend starting with the Senescence Marker Antibody Sampler Kit, which contains several of these fundamental markers, making it perfect for beginning to identify your senescent cells.
 |
Explore the Senescence Signaling Pathway Diagram to explore key protein targets and CST products. |
You can also refer to our interactive DNA Damage Response (DDR) pathways to help identify relevant targets.
Select References
- Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends in Cell Biology. 2018;28(6):436-453. doi:10.1016/j.tcb.2018.02.001
- LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res. 2014;12(2):167-183. doi:10.1158/1541-7786.MCR-13-0350
- He S, Sharpless NE. Senescence in Health and Disease. Cell. 2017;169(6):1000-1011. doi:10.1016/j.cell.2017.05.015
- Gorgoulis V, Adams PD, Alimonti A, et al. Cellular Senescence: Defining a Path Forward. Cell. 2019;179(4):813-827. doi:10.1016/j.cell.2019.10.005
- Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol. 2012;920:613-626. doi:10.1007/978-1-61779-998-3_40
- Iwabuchi K, Bartel PL, Li B, Marraccino R, Fields S. Two cellular proteins that bind to wild-type but not mutant p53. Proc Natl Acad Sci U S A. 1994;91(13):6098-6102. doi:10.1073/pnas.91.13.6098
- Ward IM, Minn K, Jorda KG, Chen J. Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J Biol Chem. 2003;278(22):19579-19582. doi:10.1074/jbc.C300117200
- Wang B, Matsuoka S, Carpenter PB, Elledge SJ. 53BP1, a mediator of the DNA damage checkpoint. Science. 2002;298(5597):1435-1438. doi:10.1126/science.1076182
- Lawless C, Wang C, Jurk D, Merz A, Zglinicki Tvon, Passos JF. Quantitative assessment of markers for cell senescence. Experimental Gerontology. 2010;45(10):772-778. doi:10.1016/j.exger.2010.01.018