Foundational approaches for studying protein function in living models—gene editing and RNA interference (RNAi)—can cause unintended, non-specific defects, result in compromised phenotypes, or be difficult to implement in certain cell lines. However, protein degradation doesn’t have to be complicated. Faster, easier protein knockdown is possible without the need to change your laboratory setup or invest in new training or equipment.
In this blog, we review an antibody-based method for protein knockdown called Trim-Away1 that can help speed your research. This protein degradation method can be applied to any endogenous protein without prior modification, uses antibodies that are widely available, and can be performed on a wide range of cell types.
How Does the Trim-Away Method Work?
Using a cell’s existing protein degradation machinery and antiviral reaction, the Trim-Away technique leverages target-specific antibodies to knock down unmodified native proteins within minutes. The key component in this process is the intracellular E3 Ubiquitin ligase TRIM21, which binds to the Fc-region of the antibody and is ubiquitinated to the point that it is degraded by the proteasome, along with the bound antibody and its specific target. By taking advantage of existing cellular processes, this technique minimizes the risk of non-specific defect accumulation and preserves the cell phenotype.
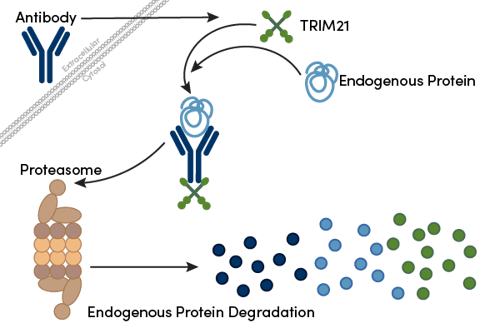 Figure 1: Schematic of the Trim-Away process. After the antibody enters the cell, it binds to its target via the epitope and TRIM21 at the Fc-region. This induces ubiquitination and degradation of the antibody-target-TRIM21 complex in the proteasome.
Figure 1: Schematic of the Trim-Away process. After the antibody enters the cell, it binds to its target via the epitope and TRIM21 at the Fc-region. This induces ubiquitination and degradation of the antibody-target-TRIM21 complex in the proteasome.
Advantages of Trim-Away for Protein Knockdown
The Trim-Away technique offers several key advantages over existing methods of protein knockdown, making it an optimal research tool that can be established in the lab with little time investment. The approach is especially useful when working with model systems where DNA knockout or RNA-based knockdown methods are limited or do not work, such as with primary cells, oocytes, and slow-proliferating cells.
Advantages of Trim-Away:
- Highly specific
- Able to target post-transcriptional modifications
- No genetic modification necessary
- Easy setup, no additional technical expertise needed
- Easy to validate
- Fast results
- TRIM21 has no housekeeping function, so normal cell function is not disrupted
With this in mind, long-lived proteins that were previously not targetable are now prime targets for knockdown experiments. This emerging approach has already shown its potential in developmental biology, tissue engineering, immunology, and target discovery, where it has been successfully used to knock down targets of interest in bovine, murine, and human embryos2. Additionally, it has been shown that this method has the potential to deplete specific post-translational modifications on a target without altering the total protein level1.
The setup for using Trim-Away is relatively simple and the experiment can be conducted on the same day, as a successful depletion occurs within a few minutes. Below are a few guidelines to keep in mind when planning your experiment.
Criteria for Antibody Selection
To achieve targeted, specific knockdown using Trim-Away in your cells, you need a highly specific antibody that will bind only to the target of interest. The antibodies that will have the best chance for success are immunoprecipitation-validated antibodies because they recognize the native structure of the proteins, which is the most important requirement for this method. The antibody must be in a glycerol-, BSA-, and azide-free formulation, since other chemicals can influence cell viability and interfere with the activation of pathways.
Nearly all monoclonal antibodies from Cell Signaling Technology (CST) are available in a compatible carrier-free formulation, either ready-to-ship or as custom products. CST rigorously validates each antibody according to the Hallmarks of Antibody Validation™ to ensure the functionality, specificity, and sensitivity of an antibody in any given assay.
Suitable Transfection Methods
Electroporation and microinjection are the two most commonly used methods to perform the transfection. Microinjection can be more effective in embryos, while electroporation works better in cell lines. Besides these, some research labs were able to optimize and make the technique work using classical chemical transfection methods3,4.
Measuring the TRIM21 Level in Your Model System
Before you start, we recommend checking the TRIM21 level in your model system. You can either use a specific expression database such as The Human Protein Atlas and BioGPS or you can perform a quick qPCR/western blot (WB) experiment.
If your specific cell type is one of the few not producing enough endogenous TRIM21, recombinant TRIM21 can be added to the transfection mix containing the antibody, to be internalized together.
Validating the Final Results
To confirm the success of the target protein knockdown, you can run a WB. A straightforward first experiment to set the Trim-Away method up in your lab is to use a GFP cell line and deplete it with an anti-GFP antibody, which allows you to watch the degradation process in real time using fluorescence microscopy.
Select References:
- Clift D, So C, McEwan WA, James LC, Schuh M. Acute and rapid degradation of endogenous proteins by Trim-Away. [published correction appears in Nat Protoc. 2018 Nov 30;:]. Nat Protoc. 2018;13(10):2149-2175. doi:10.1038/s41596-018-0028-3.
- Gerri C, McCarthy A, Alanis-Lobato G, et al. Initiation of a conserved trophectoderm program in human, cow and mouse embryos. Nature. 2020;587(7834):443-447. doi:10.1038/s41586-020-2759-x
- Clift D, McEwan WA, Labzin LI, et al. A Method for the Acute and Rapid Degradation of Endogenous Proteins. Cell. 2017;171(7). doi:10.1016/j.cell.2017.10.033
- Weir E, McLinden G, Alfandari D, Cousin H. Trim-Away mediated knock down uncovers a new function for Lbh during gastrulation of Xenopus laevis. Developmental Biology. 2021;470:74-83. doi:10.1016/j.ydbio.2020.10.014






