Did you know June is Cancer Immunotherapy Research Awareness Month? This year, we’ll celebrate 10 years since cancer immunotherapy was recognized as the “breakthrough of the year” by Science magazine in 2013. Over 30 cancers are treatable with immunotherapy and the goal of the awareness month is to increase this number further and bring life-saving therapies to even more cancer patients.
How does immunotherapy target cancer?
Traditional cancer treatments that are focused on eliminating cancer cells, like radiation therapy and chemotherapy, can have debilitating physical and emotional side effects and often have limited success with metastatic cancers. These types of treatments also do not often result in a long-term cure, as patients can come out of remission or become resistant to the treatment.
Immunotherapy, which primes a patient’s own immune system to recognize and attack cancer cells, provides an alternative approach to eliminating cancer. Using a different paradigm increases survival rates for cancers that have poor clinical outcomes using traditional approaches, such as metastatic melanoma or pancreatic ductal adenocarcinoma. Immunotherapies also have the added benefit of potentially achieving long-term remission since the immune system can recognize cancer cells if they reoccur, resulting in durable—and potentially permanent—cancer protection.
Immunotherapies play a big role in the Biden Administrations’ Cancer Moonshot program, the goal of which is to “reduce cancer death rates by 50% over the next 25 years and improve the experience of living with and surviving cancer.” As part of this program, congress has invested $1.8B to fund research, including immunotherapies, as well as patient outreach tools. At the Cancer Research Institute, $400M has been invested in immunotherapy research, while the National Cancer Institute continues to support immunotherapy by funding the continuum from basic scientific research to clinical research applications.
How many types of immunotherapies exist today?
Currently, there are multiple types of immunotherapy:
- Checkpoint Inhibitors: Block immune checkpoints that cancers use to evade the immune system.
- Immunomodulators: Stimulate the body’s immune response to effectively fight cancer. Immunomodulators used to treat cancer include cytokines, agonists, and adjuvants.
- Adoptive Cell Therapy: Isolates immune cells from the patient to expand a specific cell population or modify them to recognize a cancer antigen. Adoptive cell therapies include CAR-T therapy, tumor-infiltrating lymphocytes (TIL) therapy, engineered T-cell receptor therapy, and natural killer (NK) cell therapy.
- Targeted Antibodies: Use monoclonal antibodies against cancer-specific antigens. The antibody can be monospecific, bispecific, or conjugated to a cytotoxic drug.
- Oncolytic Virus Therapy: Modifies a virus to specifically infect and kill cancer cells without affecting non-cancerous cells. The US Food and Drug Administration (FDA) has approved IMLYGIC, a modified herpes virus that kills metastatic melanoma cells when directly injected into a tumor.
- Cancer Vaccines: Train the immune system to recognize established cancers or prevent the occurrence of cancer caused by viruses. The FDA has approved vaccines that prevent cervical and liver cancer and vaccines to treat prostate cancer, metastatic melanoma, and early-stage bladder cancer.
The list of immunotherapy types and specific therapeutics continues to grow as we learn more about T cells and how other immune cell types behave and interact with cancer cells.
What’s Hot in Cancer Immunotherapy Research?
Checkpoint Inhibitors
Recently, checkpoint inhibitors have received a lot of attention, with the 2018 Nobel Prize going to Dr James Allison and Dr Tasuku Honjo for their discovery of how cancer cells use CTLA-4 and PD-1 to evade the immune system. However, the mechanism of action for the two molecules is different. CTLA-4 is an immunosuppressive molecule normally used to keep an immune response in check by limiting T-cell priming through ligand binding competition with the costimulatory molecule CD28. Ipilimumab, the first checkpoint inhibitor approved by the FDA in 2011, inhibits CTLA-4, allowing T cells to respond to tumor antigens.
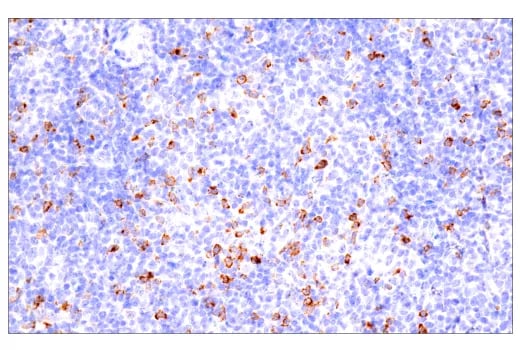
Immunohistochemical analysis of paraffin-embedded human B-cell non-Hodgkin lymphoma using CTLA-4 (E2V1Z) Rabbit mAb #53560 performed on the BOND RX Fully Automated Research Stainer by Leica Biosystems.
PD-1, on the other hand, is expressed by primed T cells and inhibits T-cell activity when it interacts with PD-L1 expressed on tumor cells and myeloid cells in the tumor microenvironment (TME). Pembrolizumab and nivolumab both block the interaction of PD-1 with PD-L1 expressed on the TME and the tumor itself, restoring the function of exhausted T cells.
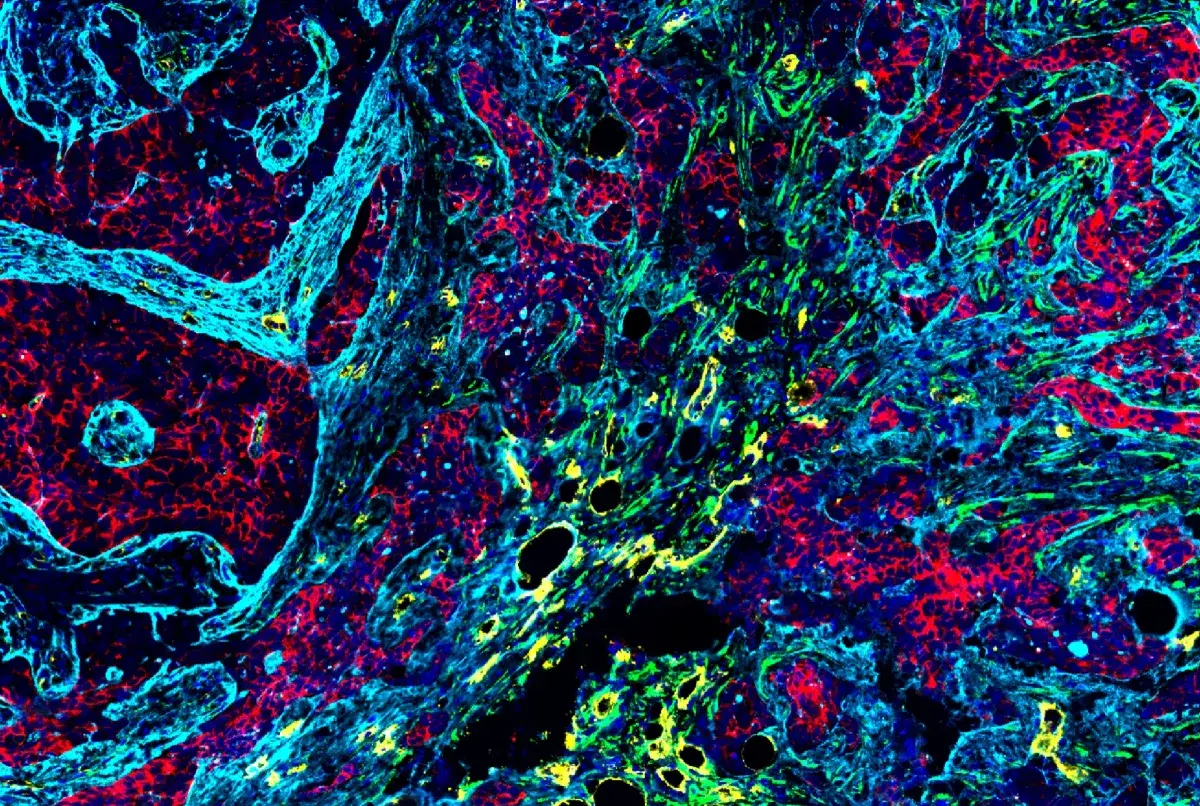
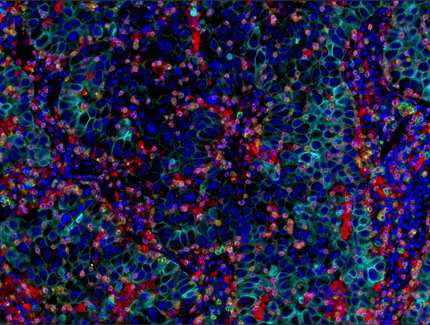 Multiplex immunohistochemical analysis of paraffin-embedded human lung adenocarcinoma using PD-1 (EH33) Mouse mAb #43248 (green), CD8α (C8/144B) mouse mAb #70306 (magenta), CD68 (D4B9C) XP® rabbit mAb #76437 (red), Pan-keratin (C11) mouse mAb #4545 (cyan), LAG3 (D2G4O™) XP® rabbit mAb #15372 (orange), and TIM-3 (D5D5R™) XP® rabbit mAb #45208 (yellow).
Multiplex immunohistochemical analysis of paraffin-embedded human lung adenocarcinoma using PD-1 (EH33) Mouse mAb #43248 (green), CD8α (C8/144B) mouse mAb #70306 (magenta), CD68 (D4B9C) XP® rabbit mAb #76437 (red), Pan-keratin (C11) mouse mAb #4545 (cyan), LAG3 (D2G4O™) XP® rabbit mAb #15372 (orange), and TIM-3 (D5D5R™) XP® rabbit mAb #45208 (yellow).
- However, only a limited population of cancer patients are successfully treated with checkpoint inhibitors. Therefore, there are efforts to evaluate the efficacy and safety of using a checkpoint inhibitor in combination with another checkpoint inhibitor or another therapy type. For example, the combination of the PD-1 inhibitor nivolumab and the CTLA-4 inhibitor ipilimumab is approved for the treatment of metastatic non-small cell lung cancer (NSCLC). In addition, relatlimab, which inhibits another immune checkpoint LAG3, was recently approved by the FDA for the treatment of unresectable or metastatic melanoma in combination with the PD-1 inhibitor nivolumab.
Targeted Antibody Immunotherapy
Targeted antibodies can be used to block targeted molecule function, induce apoptosis, or to modulate signaling pathways. For example, when the monoclonal antibody Herceptin binds to overexpressed HER2 receptors in breast and stomach cancers, it attenuates downstream signaling pathways to induce apoptosis and arrest cell proliferation. About 25% of breast cancers are HER2 positive, making it a powerful “silver bullet” in the current cancer therapy arsenal.2 Herceptin has been approved since 1998, but researchers are still looking for ways to increase efficacy or decrease cytotoxicity by investigating different epitopes or modifying the antibody to improve binding. The antibody can also be conjugated to a chemotherapy payload (antibody-drug conjugate) to specifically target the cytotoxic molecule to the tumor. Finally, bispecific antibodies are a novel approach that combines the variable region from two different antibodies into a single molecule so it can recognize epitopes from, for example, a cancer cell and a T cell. The bispecific antibody brings the T cell into close proximity to the tumor, leading to T cell activation, proliferation, and T cell-mediated killing of the cancer cells. The first bispecific antibody, blinatumomab, was approved by the FDA in 2014 to treat leukemia patients.
Most targeted antibody therapies utilize immunoglobulin G (IgG) and T cells to stimulate the immune system. However, studies looking at other immune cells and immunoglobulin types to expand the immunotherapy repertoire are ongoing. For example, studies using immunoglobulin E (IgE) targeted against chondroitin sulfate proteoglycan 4 (CSPG4), which is found on up to 70% of melanomas, have shown promising results in mice.3 IgE antibodies normally respond when presented with an allergen like pollen. Some, but not all, potential advantages of an IgE-based therapeutic compared to an IgG-based therapeutic include a higher affinity for FceRI receptors, the lack of inhibitory receptors for IgE, the ability to elicit a reaction with different effector cells compared to IgG, lower endogenous levels in the blood and therefore less receptor binding competition, and its ability mediate antibody-dependent cell-mediated cytotoxicity (ADCC) without complement-mediated cytotoxicity (CDC).4
Adoptive Cell Therapy
Adoptive cell therapy is a form of personalized medicine that expands or modifies immune cells from a patient or donor ex vivo so that the immune cells are able to recognize and kill tumor cells. The modified cells are then reinfused into the patient. Often referred to as “living drugs,” examples of adoptive cell therapy include tumor-infiltrating lymphocytes (TILs) and chimeric antigen receptor (CAR) T-cell therapy.5
TILs are blood cells that recognize and have penetrated the tumor but have been immunosuppressed by signals from the tumor and TME. A group of TILs recognizing multiple cancer antigens are isolated from a tumor biopsy and expanded, before being infused in the patient to seek and destroy tumors. The efficacy and safety of TILs, used in combination with Interleukin 2 (IL-2) and chemotherapy, to treat melanoma and other solid tumors have been extremely promising.5 However, it can take months to grow the required number of TILs for successful treatment, time that not all patients have, and some isolated TILs are difficult to grow or do not demonstrate a strong effector response. Combination therapies using TILs with checkpoint inhibitors are currently being studied. The FDA has also granted IND clearance for an engineered TIL, where the TIL has been modified to be effective without IL-2, to increase the number of patients who can benefit from this promising approach.
CAR-T therapy is the most heavily pursued type of adoptive cell therapy due to its proven high response rate and frequency of durable remissions when treating aggressive leukemias and lymphomas. CAR-T cells are immune cells that have been collected from a patient and engineered to express surface receptors that recognize tumor antigens and induce tumor cell death. The FDA has approved six CAR-T therapies, which target acute lymphoblastic leukemia in children and B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, and multiple myeloma in adults. However, there is an unmet need for successful CAR-T therapies for solid tumors due to challenges that include identifying tumor-specific antigens, the immunosuppressive nature of the TME, and CAR-T associated toxicities.6 Considerable effort to find approaches to mitigate the role of the TME, such as treating patients with CAR-T cells in combination with checkpoint inhibitor drugs or engineering CARs resistant to immunosuppressive signals specifically in the TME, is ongoing.
Another limitation to the widespread use of CAR-T therapies is the time it takes to isolate the T cells, engineer them to express the CAR, and validate CAR surface expression. Unfortunately, monitoring CAR surface expression can be complicated if detection reagents with the required specificity are hard to find. One method to avoid this issue is to validate the CAR-T in an antigen-independent fashion using anti-CAR linker antibodies that recognize the linker sequence between the variable heavy and variable light domains on the scFv.
Cancer Vaccines
The urgency required to develop a COVID-19 vaccine led to breakthroughs that, along with our better understanding of the immune system, are now being applied to cancer vaccines.7 An initially promising therapeutic approach that was all but abandoned in the 2010s, therapeutic vaccines to promote an immune response against castration-resistant prostate cancer and high-risk, on-muscle-invasive bladder cancer (NMIBC) have now been approved by the FDA.7 The advent of nucleic acid vaccine technology has addressed challenges like manufacturing time and the conflict between using live-attenuated vaccines in immunocompromised patients. Preventative vaccines have also been developed, most notably vaccines against human papillomavirus (HPV) to protect against cervical cancers, as well as against hepatitis B virus that can protect against liver cancer. Preventative vaccines for non-viral cancers are not in the clinic yet. However, researchers are investigating whether improved diagnostic and screening tools can be used in combination with therapeutic vaccines to promote anti-cancer immunity before the cancer progresses to an advanced stage.
Promising Future for Oncology Research
While immunotherapy has given patients more treatment options, it is by no means the one-shot, cure-all for cancer. Using traditional therapies like surgery, chemotherapy, and radiation therapy, in combination with the immunotherapy options described above, will give clinicians the ability to develop effective treatment strategies for each patient.
Novel and exciting breakthroughs also continue to be explored. For example, recent studies suggest that fine-tuning the gut microbiome can improve a patient’s ability to respond to treatments. When researchers seeded the colons of mice with microbiota from cancer patients, they found changes in PD-L2 and RGMb protein expression that correlated with treatment responsiveness.8 Additionally, treating the mice with broad-spectrum antibiotics to kill gut bacteria decreased their ability to respond to anti-PD-1 immunotherapy. This suggests that seeding the colonization of a patient’s gut can increase the number of patients that respond to immunotherapy.
Immunotherapies have changed the oncology treatment landscape, giving clinicians more therapeutic options to choose from. As we learn more about the immune system and think of innovative strategies to exploit regulatory mechanisms, the number of options will only grow—bringing us closer to the day when cancers can be prevented and metastatic cancers cured.
Want to Learn More? Check out these CST Tools and References
- Poster: Immuno-Oncology Signaling Pathways
- Resource Page: Pivotal Cancer Immunology Targets
- Resource Page: CAR-T Therapy Research Tools: Discover Efficacious Therapies Faster
- Blog: Anti-CAR Linker mAbs
- Brochure: Solutions for Therapeutic Discovery
Select References
- Puhr HC, Ilhan-Mutlu A. New emerging targets in cancer immunotherapy: the role of LAG3. ESMO Open. 2019;4(2):e000482. Published 2019 Mar 12. doi: 10.1136/esmoopen-2018-000482.
- Costa RLB, Czerniecki BJ. Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer. 2020;6:10. Published 2020 Mar 12. doi:10.1038/s41523-020-0153-3
- Chauhan J, et al. Anti-cancer pro-inflammatory effects of an IgE antibody targeting the melanoma-associated antigen chondroitin sulfate proteoglycan 4. Nat Commun. 2023;14(1):2192. Published 2023 Apr 25. doi: 10.1038/s41467-023-37811-3.
- Leoh LS, Daniels-Wells TR, Penichet ML. IgE immunotherapy against cancer. Curr Top Microbiol Immunol. 2015;388:109-49.
- Kirtane K, Elmariah H, Chung CH, Abate-Daga D. Adoptive cellular therapy in solid tumor malignancies: review of the literature and challenges ahead. J Immunother Cancer. 2021;9(7):e002723. doi:10.1136/jitc-2021-002723.
- Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. Published 2021 Apr 6. doi:10.1038/s41408-021-00459-7.
- Grimmett E, Al-Share B, Alkassab MB, Zhou RW, Desai A, Rahim MMA, Woldie I. Cancer vaccines: past, present and future; a review article. Discov Oncol. 2022;13(1):31. Published 2022 May 16. doi:10.1007/s12672-022-00491-4
- Park JS, Gazzaniga FS, Wu M, et al. Targeting PD-L2-RGMb overcomes microbiome-related immunotherapy resistance [published correction appears in Nature. 2023 Jun 1;:]. Nature. 2023;617(7960):377-385. doi:10.1038/s41586-023-06026-3
Sarah Klein, PhD, Associate Director, Multiplex Assay Development at Cell Signaling Technology, contributed to the writing and review of this article.
23-CAN-85660