Stress granules (SGs) are an important cellular defense mechanism that form in response to stress, helping cells pause protein production and protect key transcripts. Normally, these granules dissolve once the stress is gone. However, when this process breaks down, proteins may clump together and form toxic aggregates.
 |
Explore related antibodies and sampler kits from CST: • G3BP1 (E9G1M) XP® Rabbit mAb #61559 |
This post explores how the graules are formed, the proteins that localize to them and can be used as key markers, as well as their role in various neurodegenerative diseases.
What are stress granules?
Originally discovered in tomato cells, stress granules are small (0.1 – 4 µm), irregularly shaped foci that form in the cytoplasm in response to various cellular stressors. They contain a complex mixture of proteins and ribonucleotides, and their formation is a conserved process that is shared among many species, from yeast to humans. SG assembly is believed to be a protective adaptation in response to stress, whereby protein translation is halted, untranslated mRNA transcripts are sequestered, and the translation of stress response transcripts is prioritized. The formation of SGs is transient and they are quickly disassembled upon the removal of stressors.
The following time-lapse video highlights this dynamic process in detail:1
Stress granule assembly during NaAsO2 stress. U-2 OS cells expressing GFP-G3BP1 were exposed to NaAsO2 (0.5 mM) and imaged for 20 minutes. Images were acquired at 20-second intervals on a spinning disk confocal microscope using a 100X objective.
SGs are a type of biomolecular condensate, also referred to as a membraneless organelle (MLO). Other well-studied MLOs include the nucleolus, Cajal bodies, nuclear speckles, and processing bodies (P-bodies). MLOs are subcellular foci that compartmentalize in the absence of a double membrane and typically consist of RNA-binding proteins (RBPs) and ribonucleotides. Many of the RBPs recruited to MLOs contain intrinsically disordered regions (IDRs), which are low-complexity, unstructured regions rich in glycine and uncharged polar residues.
Due to their unique chemistry, IDRs can form numerous weak electrostatic interactions with one another, allowing IDR-containing proteins to associate and separate into distinct fluid droplets in the cytosol. This separation into fluid droplets, more commonly referred to as liquid-liquid phase separation (LLPS), is believed to be the driving force behind the formation of SGs and most other MLOs. To better visualize LLPS, think of oil separating into droplets within a glass of water.
How are stress granules formed?
As mentioned, SGs form in response to various biotic and abiotic stressors that, in turn, lead to phosphorylation of the eIF2 protein on serine 51. This well-established phosphorylation event is mediated by four different upstream kinases, depending on the type of stress:
- PKR for viral infection
- PERK for ER stress
- GCN2 for starvation/nutrient deprivation
- and HRI for oxidative or osmotic stress.2
Once phosphorylated, eIF2 can no longer exchange GDP for GTP, resulting in stalled protein translation and exposure of the 40S small ribosomal subunit interface, untranslated mRNA, and translation initiation factors. This exposed preinitiation complex (PIC) serves as a “seed” for the recruitment of SG-associated proteins and ultimately, SG assembly.3
Key Markers: Which proteins localize to stress granules?
Over 400 different proteins have been identified that localize to stress granules.4 For many of these proteins, recruitment is dependent upon multiple factors, including the type of stress induced, the disease state, and the cell line/tissue being investigated.
Here, we highlight a few of the major proteins necessary for SG assembly:
G3BP1 and G3BP2
One of the first proteins recruited to sites of stalled mRNA translation is G3BP1, a 55 kDa RBP originally thought to bind to RasGAP. However, we now know that G3BP1 is one of the key mediators of SG assembly, if not the key mediator itself. In the cytosol, G3BP1 interacts with untranslated mRNA through its RNA recognition motif (RRM) located within amino acids 340-415.
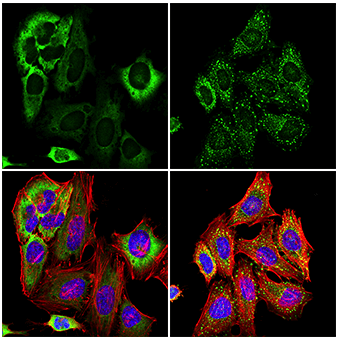 Immunofluorescent analysis of HeLa cells, untreated (left) or treated with sodium arsenite (500 μM, 30 min; right), using G3BP1 (E9G1M) XP® Rabbit mAb (green). Actin filaments were labeled with DyLight 554 Phalloidin #13054 (red). Samples were mounted in ProLong Gold Antifade Reagent with DAPI #8961 (blue). Note the translocation of G3BP1 and formation of stress granules after serum starvation or sodium arsenite treatment.
Immunofluorescent analysis of HeLa cells, untreated (left) or treated with sodium arsenite (500 μM, 30 min; right), using G3BP1 (E9G1M) XP® Rabbit mAb (green). Actin filaments were labeled with DyLight 554 Phalloidin #13054 (red). Samples were mounted in ProLong Gold Antifade Reagent with DAPI #8961 (blue). Note the translocation of G3BP1 and formation of stress granules after serum starvation or sodium arsenite treatment.
Once bound to RNA, G3BP1 recruits and interacts with a variety of other SG-associated proteins through its multiple IDRs, which also promote phase separation. Transient overexpression of G3BP1 alone is enough to induce SG formation, even in the absence of cellular stressors, highlighting its critical function in this process.5,6 Reduced levels of G3BP1 cause a decrease in the number and size of SGs formed in response to stress; however, double knockout mutations of both G3BP1 and its paralog, G3BP2, completely abolish arsenite-induced SG formation.5,7,8,9 Although less is currently known about G3BP2, it is believed to share largely redundant functions with G3BP1 regarding SG assembly.8
TIA-1 and TIAR
TIA-1 and TIAR are another pair of related RBPs that are commonly associated with SGs. Upon stress induction, TIA1/R are translocated from the nucleus to cytosolic SGs, where they are believed to form the SG “core” along with G3BP1/210. TIA1/R contains three N-terminal RRMs that interact with untranslated mRNA, and a C-terminal prion-like domain (PLD) that is necessary for phase separation and SG formation.11 Like G3BP1, overexpression of TIA1 alone is enough to induce SG formation in the absence of stress.10
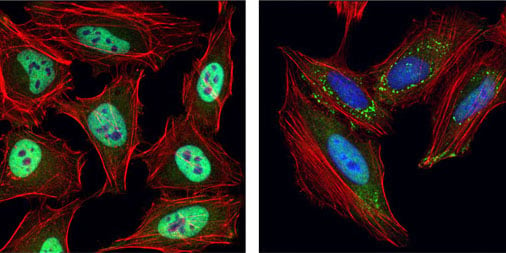
IF analysis of HeLa cells, untreated (left) or UV-treated (right), using TIAR (D26E4) Rabbit mAb #8611 (green). Actin filaments were labeled with DyLight 554 Phalloidin #13054 (red). Blue = DRAQ5 #4084 (fluorescent DNA dye). Note the translocation of TIAR and formation of stress granules upon UV treatment.
Additional Protein Markers
Many other notable proteins are recruited to and are involved in the regulation of SGs, including FMR1/FMRP, PABPC1, ataxin-2, caprin1, and UBAP2L. UBAP2L is of particular interest because it may play a role in SG formation upstream of G3BP1/2; this novel finding highlights the need for further research into the dynamics of SG assembly.13
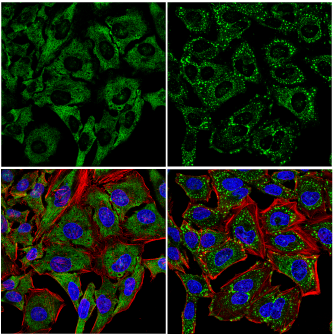
IF analysis of HeLa cells, untreated (left) or treated with sodium arsenite (500 µM, 30 min; right), using UBAP2L (E5X4E) Rabbit mAb (green). Actin filaments were labeled with DyLight 554 Phalloidin #13054 (red). Blue = DRAQ5 #4084 (fluorescent DNA dye). Note the translocation of UBAP2L and formation of stress granules sodium arsenite treatment.
Stress Granules in Neurodegeneration
Under normal conditions, the formation of stress granules is a transient, reversible process; when stress has dissipated, SG disassembly is carried out by the recruitment of multiple autophagy-promoting proteins such as VCP and SQSTM1. However, due to the high concentration of protein-protein interactions, SGs are especially susceptible to forming toxic protein aggregates if disassembly is disrupted. This transition of SGs from soluble, liquid-phase mixtures to insoluble, solid-phase aggregates can act as a seed for the development of cytosolic inclusion bodies, which is a defining characteristic of various neurodegenerative diseases.
TDP43, an RNA-binding protein involved in transcriptional regulation and exon splicing, is the major pathological protein associated with amyotrophic lateral sclerosis (ALS). Accumulation of TDP43 is also associated with Alzheimer’s disease (AD) and frontotemporal dementia (FTD). Disease-associated mutations in TDP43 can promote a myriad of changes to the biophysical properties of SGs, such as mislocalization of TDP43 from the nucleus to cytosolic stress granules, increasing the size and number of SGs, and the conversion of SGs to pathological inclusion bodies.14,15,16,17
FUS is another RNA-binding protein that plays a role in transcriptional regulation, pre-mRNA splicing, and DNA damage response. Like TDP43, pathological accumulation of FUS-containing protein aggregates has been identified in several neurodegenerative diseases. Mutations in FUS are common in ALS and FTD patients, typically occurring within the protein’s C-terminal nuclear localization sequence. These mutations cause mislocalization of the predominantly nuclear protein into the cytoplasm and consequently promote the accumulation of FUS aggregates in cytosolic stress granules.18
While it is not considered to be a conventional RBP, the microtubule-associated protein tau has also been shown to bind to RNA, as well as colocalize with several SG-associated proteins including TIA1, ataxin-2, and PABPC11.9,20 Tau can also undergo LLPS, which is enhanced in the presence of RNA; however, tau does not form RNA granules independent from SGs. This suggests that tau is recruited to SGs via interaction with mRNA and RBPs, where it can potentially form insoluble tau aggregates. Hyperphosphorylation of tau may also affect its recruitment to SGs.20
Future Research
Stress granules are a unique and fascinating physiological adaptation, allowing cells to react to changes in protein translation through a series of cell signaling events coupled with liquid-liquid phase separation. This is an exciting field of study, and future research will likely shed new light on the assembly/disassembly of SGs, novel components recruited to these foci, and their relevance to human disease. Great progress has already been made linking SGs to neurodegenerative diseases such as ALS and FTD, and potential therapies targeting SG protein misfolding and aggregation could prove useful in treating these disorders.
Additional Resources:
- Refer to our interactive Stress Granule Lifecycle diagram for additional information about stress granules.
- Interested in other MLOs? Request a copy of our Biomolecular Condensates Marker poster.
- Check out the interactive Alzheimer's Disease Signaling Pathway.
- Download the brochure: Hallmarks of Neurodegeneration: Identifying Underlying Biological Processes.
- Watch the Science webinar, Stress granules: Deciphering the connections to neurodegeneration.
Select References:
- Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R. Distinct stages in stress granule assembly and disassembly. Elife. 2016;5:e18413. Published 2016 Sep 7. doi:10.7554/eLife.18413. CC BY 4.0.
- Mahboubi H, Stochaj U. Cytoplasmic stress granules: Dynamic modulators of cell signaling and disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863(4):884-895. doi:10.1016/j.bbadis.2016.12.022
- Panas MD, Ivanov P, Anderson P. Mechanistic insights into mammalian stress granule dynamics. J Cell Biol. 2016;215(3):313-323. doi:10.1083/jcb.201609081
- Marcelo A, Koppenol R, de Almeida LP, Matos CA, Nóbrega C. Stress granules, RNA-binding proteins and polyglutamine diseases: too much aggregation?. Cell Death Dis. 2021;12(6):592. Published 2021 Jun 8. doi:10.1038/s41419-021-03873-8
- Tourrière H, Chebli K, Zekri L, et al. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160(6):823-831. doi:10.1083/jcb.200212128
- Reineke LC, Dougherty JD, Pierre P, Lloyd RE. Large G3BP-induced granules trigger eIF2α phosphorylation. Mol Biol Cell. 2012;23(18):3499-3510. doi:10.1091/mbc.E12-05-0385
- Ghisolfi L, Dutt S, McConkey ME, Ebert BL, Anderson P. Stress granules contribute to α-globin homeostasis in differentiating erythroid cells. Biochem Biophys Res Commun. 2012;420(4):768-774. doi:10.1016/j.bbrc.2012.03.070
- Matsuki H, Takahashi M, Higuchi M, Makokha GN, Oie M, Fujii M. Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells. 2013;18(2):135-146. doi:10.1111/gtc.12023
- Kedersha N, Panas MD, Achorn CA, et al. G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits [published correction appears in J Cell Biol. 2020 Jan 6;219(1):]. J Cell Biol. 2016;212(7):845-860. doi:10.1083/jcb.201508028
- Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999;147(7):1431-1442. doi:10.1083/jcb.147.7.1431
- Waris S, Wilce MC, Wilce JA. RNA recognition and stress granule formation by TIA proteins. Int J Mol Sci. 2014;15(12):23377-23388. Published 2014 Dec 16. doi:10.3390/ijms151223377
- Gilks N, Kedersha N, Ayodele M, et al. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15(12):5383-5398. doi:10.1091/mbc.e04-08-0715
- Cirillo L, Cieren A, Barbieri S, et al. UBAP2L Forms Distinct Cores that Act in Nucleating Stress Granules Upstream of G3BP1. Curr Biol. 2020;30(4):698-707.e6. doi:10.1016/j.cub.2019.12.020
- Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-133. doi:10.1126/science.1134108
- Winton MJ, Igaz LM, Wong MM, Kwong LK, Trojanowski JQ, Lee VM. Disturbance of nuclear and cytoplasmic TAR DNA-binding protein (TDP-43) induces disease-like redistribution, sequestration, and aggregate formation. J Biol Chem. 2008;283(19):13302-13309. doi:10.1074/jbc.M800342200
- Bentmann E, Neumann M, Tahirovic S, Rodde R, Dormann D, Haass C. Requirements for stress granule recruitment of fused in sarcoma (FUS) and TAR DNA-binding protein of 43 kDa (TDP-43). J Biol Chem. 2012;287(27):23079-23094. doi:10.1074/jbc.M111.328757
- Mann JR, Gleixner AM, Mauna JC, et al. RNA Binding Antagonizes Neurotoxic Phase Transitions of TDP-43. Neuron. 2019;102(2):321-338.e8. doi:10.1016/j.neuron.2019.01.048
- Vance C, Scotter EL, Nishimura AL, et al. ALS mutant FUS disrupts nuclear localization and sequesters wild-type FUS within cytoplasmic stress granules. Hum Mol Genet. 2013;22(13):2676-2688. doi:10.1093/hmg/ddt117
- Vanderweyde T, Apicco DJ, Youmans-Kidder K, et al. Interaction of tau with the RNA-Binding Protein TIA1 Regulates tau Pathophysiology and Toxicity. Cell Rep. 2016;15(7):1455-1466. doi:10.1016/j.celrep.2016.04.045
- Wolozin B, Ivanov P. Stress granules and neurodegeneration. Nat Rev Neurosci. 2019;20(11):649-666. doi:10.1038/s41583-019-0222-5







