In November, nearly 25,000 neuroscientists from all over the world gathered once again for the Society of Neuroscience (SfN) Meeting 2022 in San Diego after three years of canceled or virtual meetings due to the COVID-19 pandemic. During the five-day annual meeting, participants shared their latest findings, tools, and techniques for advancing the field of neuroscience.
As neuroscientists, we recognize the importance of making connections—and how challenging it can be to develop a strong network in a remote, virtual world. With spatial biology and computational genomics continuing to be at the forefront of neuroscience research and a key focus at the conference, the return to an in-person event was an apt reminder of the importance of proximity in making connections and building networks.

Cell Signaling Technology booth at SfN 2022.
Needless to say, it was refreshing to see fellow brain scientists in person again, to share news of recent breakthroughs across a podium instead of through a computer screen, and to develop deeper, more meaningful, and lasting connections with fellow researchers.
SfN 2022 Recap: Mixed News Surrounding Alzheimer's Disease Therapies in 2022
It was fitting that the conference came on the heels of the announcement from Biogen and Eisai that they achieved positive top-line results for their Clarity Alzheimer's disease (AD) confirmatory phase 3 clinical trial of lecanemab. The promising antibody drug, which appears to clear amyloid plaques and slow cognitive decline among AD patients, was received with some caution at the show. Neuroscientists had received similar promising news about aducanumab, which was announced with even greater fanfare but was subject to huge controversy and eventually abandoned.
Although I remain cautiously optimistic about the news of this potential therapeutic breakthrough, I suspect that real advancement will not come until the cellular and molecular basis of AD is better understood. Certainly, new tools, technologies, and more relevant model systems will accelerate discovery in this space. Most importantly, understanding how all the cells in the brain are interconnected and networked remains key to driving discovery in AD research.
Hallmarks of Neurodegeneration: Neuroinflammation
One of the hallmarks of neurodegeneration that contributes to AD is neuroinflammation, the process in which glial cells, like microglia and astrocytes, become reactive under diseased or damaged conditions. How these cells directly contribute to disease is under intense investigation, and SfN was rich with reports of new and exciting findings regarding this process. It’s difficult to summarize the hundreds of posters and talks that were presented on this hot topic, but one particular session, Alzheimer’s Disease: Glial Cells, highlighted how glia might contribute to disease pathology via altered engagement with neurons in disease conditions.
Microglia are known to eliminate excess neuronal synapses during development in a process known as synaptic pruning. What isn’t clearly understood, however, is if this process occurs under disease conditions, particularly after developmental synaptic pruning has ceased. Although investigators have speculated that glia might directly contribute to this process, evidence, particularly in humans, has been lacking.
Work from two independent groups presented at the show suggested that human synaptic loss in AD might occur via the glia. Dr Tara L. Spires-Jones’ group (presented by M. Tzioras) from the UK Dementia Research Institute, as well as Dr Teresa Gomez-Isla (presented by R.N. Taddei) of Massachusetts General Hospital, examined post-mortem brain tissue from AD patients. Using co-labeling antibody staining techniques to label synaptic proteins (like Synapsin I) and glial markers (like CD68 and GFAP), both groups observed increased synaptic puncta, likely indicative of synaptic debris taken up by glial cells, in microglia from human AD patients compared to non-diseased samples.
Surprisingly, Dr Spires-Jones’ group also observed synaptic debris in astrocytes, demonstrating for the first time that astrocytes may also contribute to disease-associated synaptic loss. Using isolated primary human microglia and astrocytes, Dr. Spires-Jones was able to correlate glial uptake of synapses with genetic risk factors and suggested an MFG-E8-dependent mechanism for engulfment.
Dr Gomez-Isla’s group observed similar synaptic engulfment by microglia and astrocytes in disease conditions and also proposed a model by which astrocytes mediate synapse elimination well before the appearance of late-stage pathological markers. Interestingly, Dr Gomez-Isla used a novel technique, expansion microscopy, which enables super-resolution imaging of tissues, such as the brain, by expanding and physically enlarging the tissue to be imaged.
Expansion microscopy is an emerging technology that may enable the discovery of key cellular and molecular mechanistic insights in AD. As always, I am optimistic the development of new technologies and tools will enable scientific discovery and progress.
New Antibody Tools for Studying Alzheimer’s Disease
This year was a special year because Cell Signaling Technology (CST) presented two posters showcasing new tools we had developed to better understand the cellular and molecular contributions of AD.
Poster: Antibodies to Investigate the TREM2 Signaling Pathway
In the first poster, presented by my colleague, Jordan Hirschfeld, we developed antibody-based tools that can be used to investigate the TREM2 signaling pathway. TREM2 is a receptor protein expressed in microglia that is genetically and mechanistically linked to AD. Investigators are targeting TREM2 for therapeutic intervention, yet signaling cascades downstream of TREM2 have yet to be fully characterized.
To facilitate the development of TREM2 modulators, we established a TREM2 signaling readout by screening potential TREM2 downstream signaling molecules. We identified phospho-Syk (pSyk) as a robust readout for TREM2 activation using a previously described TREM2-activating antibody. In order to identify our own TREM2-activating antibody, we screened a library of TREM2 extracellular domains targeting rabbit monoclonal antibodies for pSyk activation using microglia-like immortalized human and mouse cell lines (Figure 1).
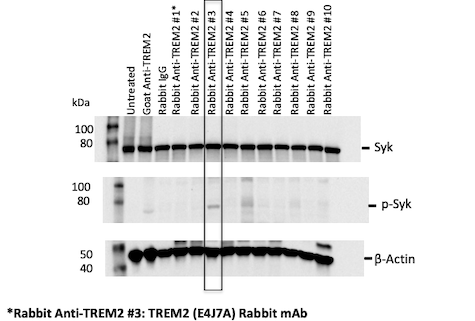 Figure 1: A library of anti-human TREM2 recombinant monoclonal antibodies was generated and screened for in vitro stimulation of TREM2 using pSyk as a readout.
Figure 1: A library of anti-human TREM2 recombinant monoclonal antibodies was generated and screened for in vitro stimulation of TREM2 using pSyk as a readout.
Using this strategy, we identified multiple human and mouse TREM2-activating monoclonal antibodies. Development of these tools, both readout and activating TREM2 antibodies, will help facilitate drug development against this important microglia-based therapeutic AD target.
Poster: Antibodies to Characterize Microglial Activation States
How do microglia directly or indirectly contribute to AD initiation and progression? This is an area that remains under intense investigation. What is clear, based on multiple RNA-seq studies of the last five years, is that microglia exist in distinct molecular states in the context of disease. However, transcriptomic profiling of cells, although powerful, has its limitations. For example, RNA profiles may not correlate faithfully with protein expression, which ultimately drives microglia function. Moreover, the spatial context of protein expression is an important factor in understanding the relationship between microglia and disease. For example, are the translated proteins linked to RNA targets associated with disease similarly upregulated in microglia? Are they linked to the proximity of pathological hallmarks of disease, for example, amyloid-beta (Aβ) plaques in AD?
In order to investigate these questions, we presented a second poster titled “Development of novel rabbit monoclonal antibodies to characterize microglial activation states in murine models of Alzheimer’s disease,” which describes antibody-based tools developed to stain proteins that could mark disease-associated microglia. We highlighted one particular antibody against Cathepsin D, a lysosomal aspartyl protease involved in protein degradation. The gene that encodes Cathepsin D is upregulated in microglia in mouse models of AD. Similarly, when staining microglia using an antibody for Cathepsin D and a multiplex immunofluorescence strategy, we observed Cathepsin D-positive microglia surrounding amyloid plaques, suggesting that this new tool can be used to label reactive microglia that may directly contribute to Alzheimer’s disease progression (Figure 2).
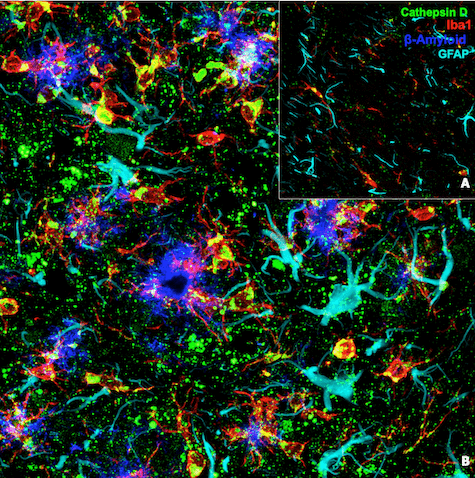
Figure 2: Confocal immunofluorescent analysis of brain from a wild-type mouse (A) and an amyloid mouse model of AD (B) using Cathepsin D (E7Z4L) XP® Rabbit mAb #88239 (green), Iba1/AIF-1 (E4O4W) XP® Rabbit mAb (Alexa Fluor® 647 Conjugate) #78060 (red), GFAP (cyan), and β-Amyloid (blue).
Future opportunities include profiling multiple molecular signatures. Moreover, new technologies will emerge to examine cellular morphologies that may correlate with cellular function.
The Quest for New Insights, Technologies, and Tools Continues
After nearly three years of missing key conferences, I welcomed the opportunity to witness the interplay between the quest for new discoveries and insights, technology, and tools in the world of neuroscience. Now, more than ever, the world of science is interconnected in ways that we cannot imagine. SfN is a special conference because it captures what is so fascinating about the brain—how do the networks of neurons, including non-neuronal cells like glia, work together to drive complex human behavior and disease?
I can’t wait for the next meeting in Washington, DC in 2023, and hope to see you there!
Additional Resources
- Blog: Microglial Activation: High-Content Immunofluorescence to Study TREM2 Signaling
- eBook: Hallmarks of Neurodegeneration
Alexandra Foley, Scientific Marketing Writer and CST Blog Manager, contributed to writing this post.










