With Alzheimer’s disease (AD) set to affect more than 7.1 million people over 65 worldwide by 2025, it’s essential to develop tools that can facilitate new breakthroughs and make a difference against this devastating neurodegenerative condition.1 Detection and assessment of AD currently relies on costly and invasive cerebrospinal fluid (CSF) biomarker measurements and neuroimaging techniques, and researchers have long awaited the availability of a reliable blood-based AD biomarker to enable early detection, monitoring, and research. In recent decades, it’s become increasingly clear that phosphorylated tau proteins just may be the key.2
Among the many phosphorylation sites on human tau, threonine 217 (p-tau217) stands out. Phosphorylation at this site holds promise because its levels increase in autosomal dominant AD patients,3 enabling the discrimination of AD patients from non-AD patients compared to other tau phosphorylation sites.4,5 It's also been shown that blood plasma levels of p-tau217 are elevated in the early stages of AD, suggesting that it could be a promising biomarker to aid in longitudinal AD research and drug development, as well as diagnosis, disease monitoring, and patient care.6,7
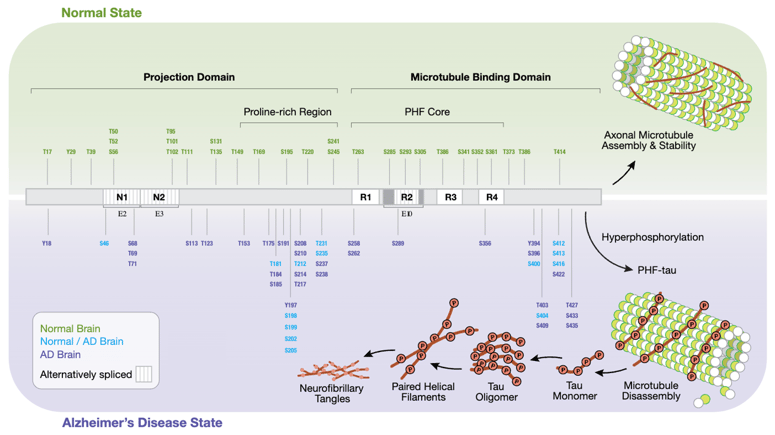
Tau hyperphosphorylation in Alzheimer’s disease. Tau, a microtubule-associated protein (MAP), exhibits altered and increased phosphorylation patterns due to changes in tau kinase/phosphatase activity. This impairs tau’s ability to stabilize microtubules, leading to increased microtubule catastrophe and impaired cargo trafficking. Pathological hyperphosphorylation of tau increases its susceptibility to aggregate into paired helical filaments, which form into large intracellular neurofibrillary tangles (NFTs), a noted hallmark of AD visible in diseased tissue. Microtubule destabilization and NFTs both contribute to the neurotoxicity and neurodegeneration associated with AD and tauopathies. Download the poster using the link at the end of this post to view the high-res version of this diagram.
I worked with researchers in our neuroscience department to develop a monoclonal antibody for detecting the threonine 217 phosphorylation site on tau. When measuring fluid-based biomarkers like p-tau217, it’s important that the antibody is highly specific and sensitive to the target, ensuring it works well in experiments like enzyme-linked immunosorbent assays (ELISAs), single-molecule arrays, and immunoprecipitation mass spectrometry (IP-MS).
Our resulting monoclonal antibody clone accurately detects p-tau217 in human/rodent tissue and plasma and exhibits superior avidity and binding kinetics compared with a commercially available competitor antibody. The clone, E9Y4S, is offered in the following CST products:
Keep reading to learn more about the development and validation of our phospho-tau antibody products and their applicability for AD research.
Developing a Monoclonal Antibody Clone for P-Tau217
Developing an antibody that is sensitive and specific to phosphorylated tau at threonine 217 took deep scientific expertise, time, care, resources, and the collaborative effort of many people. During the process, we evaluated a library of nearly one thousand antibody clones for p-tau peptides and tau-expressing tissue (+/- λ phosphatase) using ELISA/Dot Blots and western blot analysis. We identified clone E9Y4S, which we expanded and recombinantly expressed to examine and characterize further.
Determining Antibody Specificity
At CST, we take antibody specificity seriously, and we’re proud to be the vendor with the most-cited research antibodies year after year. We know it’s essential that all of our antibodies perform accurately and reliably so that you can trust the results and move your research projects forward. Ensuring that our p-tau217 antibody products are specific was not achieved via one experiment—it was done in many different ways using multiple techniques.
The following experiments were conducted to examine different types of specificity, including phospho-specificity, site-specificity, and target specificity:
- Phospho-specificity, i.e., specificity for the post-translational modification (PTM), was confirmed by treating mouse brain lysates with λ phosphatase, which removes phosphorylation on the protein and results in the loss of the antibody signal.
- Site-specificity was determined through dot blot assays using synthetic peptides for multiple individual phosphorylation sites. This confirms the antibody’s ability to detect phosphorylation at threonine 217.
- Target specificity was demonstrated by comparing wild-type mouse brain tissue with tau knockout models, ensuring the antibody specifically binds to tau.
Additional validation was performed via IP, followed by WB, to check for enrichment of the tau protein. Testing with Alzheimer's disease brain samples confirmed the antibody's applicability in real-world research scenarios. The enhanced signals seen in these models further demonstrate the antibody’s relevance and specificity.
The following figures show some of the experiments we conducted to verify phospho-specificity, site-specificity, and target specificity for clone E9Y4S:
 Determining phospho-specificity, site-specificity, and target specificity. (A) Comparison of mouse brain lysate -/+ λ phosphatase treatment shows E9Y4S is phospho-specific. (B) Comparison of wild-type (WT) mouse brain to a confirmed tau -/- mouse brain shows target specificity to tau. (C) IP of Tau Thr217 in mouse brain tissue extracts shows enrichment compared to the input. (D) Target specificity confirmation using a research-relevant model system, where enhanced p-tau217 signal was detected in AD cortex/hippocampus compared to the control cortex. (E) A peptide dot blot immunoassay demonstrates specificity to threonine 217 by comparing against a non-phosphorylated control and surrounding phosphorylated sites.
Determining phospho-specificity, site-specificity, and target specificity. (A) Comparison of mouse brain lysate -/+ λ phosphatase treatment shows E9Y4S is phospho-specific. (B) Comparison of wild-type (WT) mouse brain to a confirmed tau -/- mouse brain shows target specificity to tau. (C) IP of Tau Thr217 in mouse brain tissue extracts shows enrichment compared to the input. (D) Target specificity confirmation using a research-relevant model system, where enhanced p-tau217 signal was detected in AD cortex/hippocampus compared to the control cortex. (E) A peptide dot blot immunoassay demonstrates specificity to threonine 217 by comparing against a non-phosphorylated control and surrounding phosphorylated sites.
Characterization of Antibody Affinity and Binding Kinetics
Aside from knowing that an antibody will perform in the approved assays, researchers may find it helpful to understand how strongly an antibody binds to its target epitope—i.e., the affinity of the antibody. Knowing the affinity of an antibody can be valuable in predicting how the antibody will perform in multiple assays. For example, measuring the on-off rates to determine an antibody’s kinetics can inform compatibility in pairing the antibody with others, which is important in pair-based assays like ELISA. Affinity and kinetics information may also be useful for comparing the potential performance of commercially available antibodies across vendors.
The binding kinetics of Phospho-Tau (Thr217) (E9Y4S) Rabbit mAb and a commercially available competitor monoclonal antibody were measured using bio-layer interferometry (BLI) on the Octet RED96 (Sartorius). Biotinylated Tau 217 or p-tau217 peptides were attached to High Precision Streptavidin biosensors and exposed to different concentrations of our antibody clone or the competitor monoclonal antibody, as shown in the figure below.
The higher binding and lower dissociation rates for the CST antibody clone demonstrate that our antibody has both a higher affinity for the p-tau217 peptide and more stable binding than the competitor antibody.
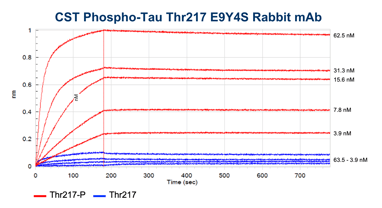
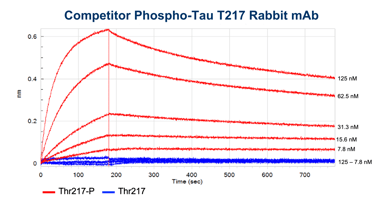
BLI data comparing the CST antibody clone (left) to a competitor monoclonal antibody (right). Binding to the p-tau217 peptide is shown in red and binding to the non-phosphorylated peptide in blue. Concentrations tested range from 250 nM to 3.9 nM. Global curve-fitting was performed using a bivalent analyte model; only the concentrations that passed that fitting are shown.
Using the Phospho-Tau (Thr217) (E9Y4S) Rabbit mAb in an ELISA
While western blot, IP, and BLI all offer useful information, the sensitivity and ease of use of an ELISA give it greater utility for testing biofluid samples. We used clone E9Y4S to develop the Phospho-Tau (Thr217) Matched Antibody Pair #70933, the PathScan® RP Phospho-Tau (Thr217) Chemiluminescent Sandwich ELISA Kit #87749, and the PathScan® RP Phospho-Tau (Thr217) Sandwich ELISA Kit #59672. The main advantage of our PathScan RP kits is that they require only 15 minutes of hands-on time and can be used to generate results in as little as 1.5 hours.
Blog: ELISA Lot-to-Lot Reproducibility: Safeguard the Quality of Your Data
Using the PathScan® RP Phospho-Tau (Thr217) Sandwich ELISA Kit #59672, we were able to detect p-Tau217 in rodent brain tissue and identify elevated p-tau217 levels in human AD brain tissue compared to non-diseased controls, as shown below.
%20Rabbit%20mAb/Evaluation%20of%20the%20PathScan%20RP%20Phospho-Tau%20(Thr217)%20Sandwich%20ELISA%20Kit%20%2359672.png?width=1148&height=600&name=Evaluation%20of%20the%20PathScan%20RP%20Phospho-Tau%20(Thr217)%20Sandwich%20ELISA%20Kit%20%2359672.png)
Evaluation of the PathScan® RP Phospho-Tau (Thr217) Sandwich ELISA Kit #59672. (A) The sensitivity of #59672 was measured using recombinant Tau protein at indicated concentrations. (B) The phospho-sensitivity of #59672 in brain tissue was measured using mouse brain tissue at indicated lysate protein titrations (+/- λ phosphatase). (C) Relative levels of p-Tau217 from rodent brain (+/- λ phosphatase) and human AD/Control brain were measured using #59672.
The PathScan® RP Phospho-Tau (Thr217) Sandwich ELISA Kit #59672 also allows for the detection of elevated levels of p-tau217 in plasma from the TauP301S transgenic mouse model compared to WT controls, as shown in the figure below. In combination, these data suggest that our identified p-Tau217 ELISA pair and assay could be used to detect p-Tau217 in biofluids for research purposes.
%20Rabbit%20mAb/Verification%20of%20the%20PathScan%20Phospho-Tau%20(Thr217)%20Sandwich%20ELISA%20Kit%20%2359672%20with%20plasma%20samples.png?width=200&height=263&name=Verification%20of%20the%20PathScan%20Phospho-Tau%20(Thr217)%20Sandwich%20ELISA%20Kit%20%2359672%20with%20plasma%20samples.png)
Verification of the PathScan® Phospho-Tau (Thr217) Sandwich ELISA Kit #59672 with plasma samples. #59672 discriminates phosphorylation of tau at Thr217 in plasma from 3-month old Tau P301S mice (Tau) compared to control mice (Non-Tg). Data provided by Dr Ping-Chieh Pao and Dr Li-Huei Tsai (MIT) and used with permission.
Antibodies You Can Trust
The rigorous experiments we conduct, such as those described here, are why we can confidently guarantee that CST antibodies will perform as expected in the assays for which they are intended. Our highly sensitive and specific Phospho-Tau (Thr217) (E9Y4S) rabbit monoclonal antibody binds to p-tau217 with high avidity and strong binding kinetics, so you know you can trust your results the first time and every time.
Learn more about the experiments we conducted to validate clone E9Y4S for WB, IP, and ELISA in the poster presented at SfN:





%20Rabbit%20mAb/Evaluation%20of%20the%20PathScan%20RP%20Phospho-Tau%20(Thr217)%20Sandwich%20ELISA%20Kit%20%2359672.png?width=1148&height=600&name=Evaluation%20of%20the%20PathScan%20RP%20Phospho-Tau%20(Thr217)%20Sandwich%20ELISA%20Kit%20%2359672.png)
%20Rabbit%20mAb/Verification%20of%20the%20PathScan%20Phospho-Tau%20(Thr217)%20Sandwich%20ELISA%20Kit%20%2359672%20with%20plasma%20samples.png?width=200&height=263&name=Verification%20of%20the%20PathScan%20Phospho-Tau%20(Thr217)%20Sandwich%20ELISA%20Kit%20%2359672%20with%20plasma%20samples.png)