One of the benefits of working with immunofluorescent (IF) analysis is the ability to compare multiple protein expression patterns within a single sample. There are several approaches to multiplexing with IF.
Indirect Detection: Host-based Immunofluorescent Multiplexing
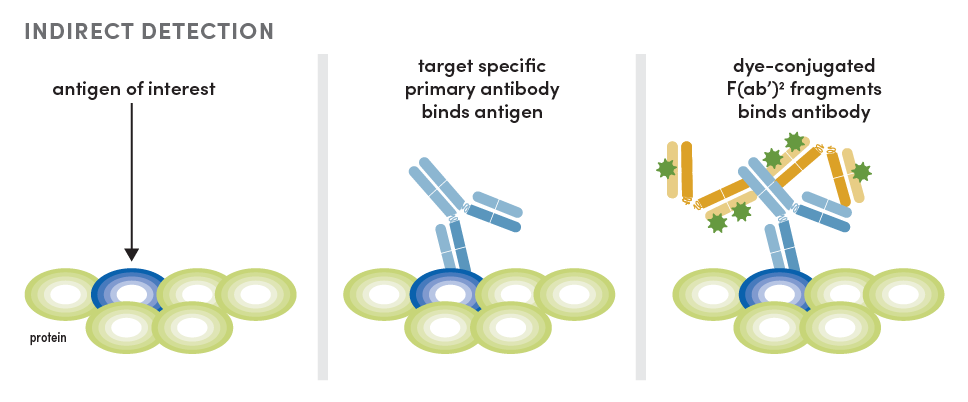
Currently, the most popular method is host-based multiplexing. In this scenario, each antibody has a unique host species that allows for independent detection of each antibody with host-specific secondary antibodies conjugated to fluorophores. This method is also called indirect detection.
Direct Detection Using Dye-Conjugated Primary Antibodies
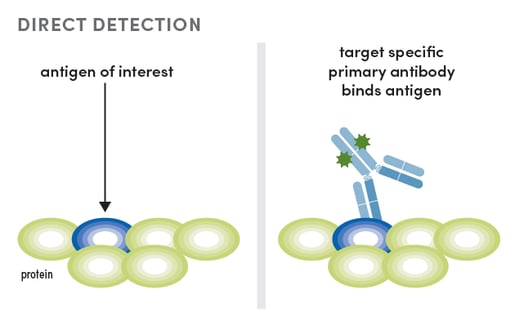
Another approach to IF multiplexing is to work with dye-conjugated primary antibodies. This method is referred to as direct detection. There are a few drawbacks to direct detection. First, experimental design is less flexible because you are working with spectral requirements for your antibodies. Additionally, conjugates tend to be dimmer than when using indirect detection. This is due to the fact that a conjugate is limited to how many fluorophores it is labeled with, while multiple secondary antibodies conjugated to fluorophores can detect a primary antibody using indirect detection.
While direct detection has drawbacks, there is also a payoff because this method is faster than host-based indirect detection. Direct detection is also more approachable for a beginner, as there is only one antibody binding step necessary to produce signal. Furthermore, the drawbacks are decreasing. There is growing interest in working with oligo-conjugated antibodies, which can greatly increase the dimensions for multiplexing and the sensitivity of detection. Unfortunately, this method is not as fast as working with dye-conjugated antibody.
Sequential Labeling Strategy for IF Multiplexing
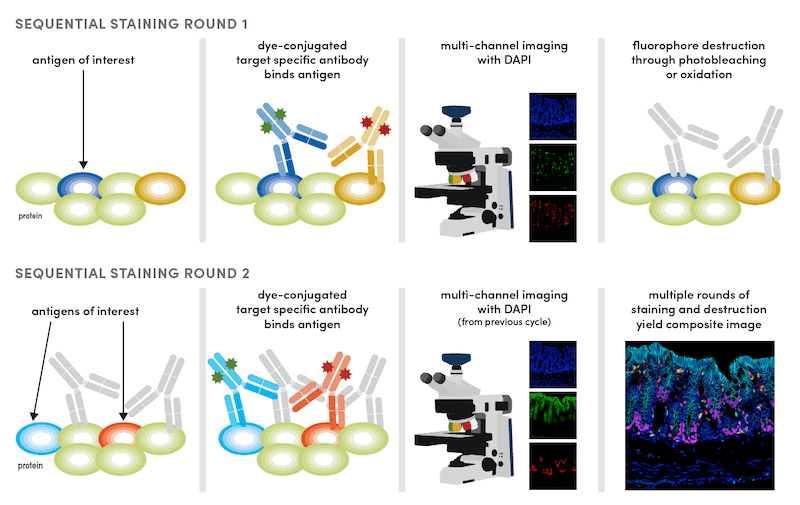
The third IF approach involves some sort of sequential labeling strategy. This may be through sequential rounds of labeling with a primary antibody, tyramide-streptavidin (TSA) detection, and antibody destruction (as is common with multiplex IHC). Alternatively, it may involve sequential rounds of labeling with conjugates, imaging, and cleavage of the dye from the antibody (a typical cyclic IF protocol).
 It may be tempting to work with only conjugates as the workflow is simpler and faster; however, there are several situations where indirect detection may be beneficial. You may need the brighter signal provided by indirect detection when examining a low-abundance target. There may be an antibody that is not available in a conjugated form or is not available conjugated to the dye needed for your experiment. In these cases, it is possible to combine host-based detection and staining with dye-conjugated primary antibodies. The most straightforward version of this approach would be to use unique hosts with the conjugate and not include a secondary antibody capable of detecting the conjugated antibody.
It may be tempting to work with only conjugates as the workflow is simpler and faster; however, there are several situations where indirect detection may be beneficial. You may need the brighter signal provided by indirect detection when examining a low-abundance target. There may be an antibody that is not available in a conjugated form or is not available conjugated to the dye needed for your experiment. In these cases, it is possible to combine host-based detection and staining with dye-conjugated primary antibodies. The most straightforward version of this approach would be to use unique hosts with the conjugate and not include a secondary antibody capable of detecting the conjugated antibody.
At CST, many of our antibodies are derived from rabbits. We have found a useful technique for multiplexing with two (or more) antibodies of the same host species. This method relies on combining dye-conjugated primaries with one unconjugated antibody of the same host species as the conjugated primaries.
The steps are as follows:
- Incubate the sample with all unconjugated primary antibodies overnight at 4° C at recommended concentrations.
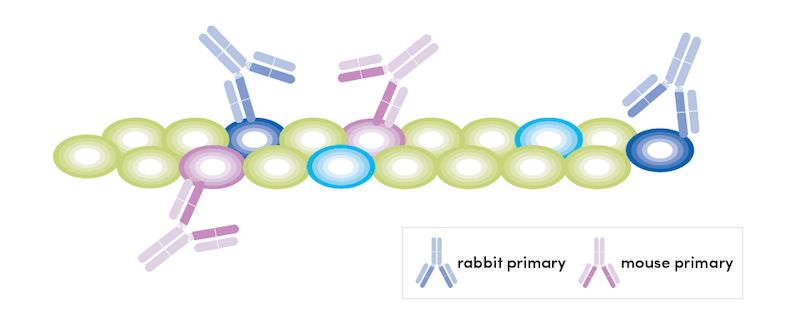
- Wash the sample three times in 1X phosphate-buffered saline (PBS).
- Detect primary antibodies with corresponding fluorophore-conjugated secondaries.
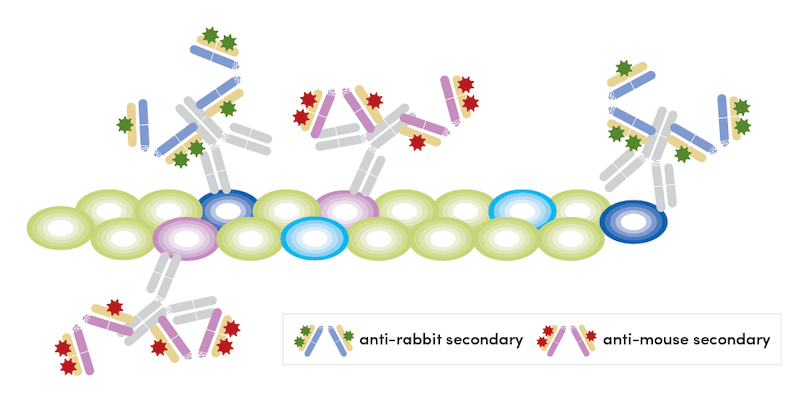
- Wash the sample three times in 1X PBS.
- Block free secondary binding sites with appropriate host unconjugated immunoglobulin in excess (10X of primary concentration) for 1 hour at room temperature. We have validated both Rabbit (DA1E) mAb IgG XP Isotype Control #3900 and Mouse (G3A1) mAb IgG1 Isotype Control #5415 to work for this purpose.
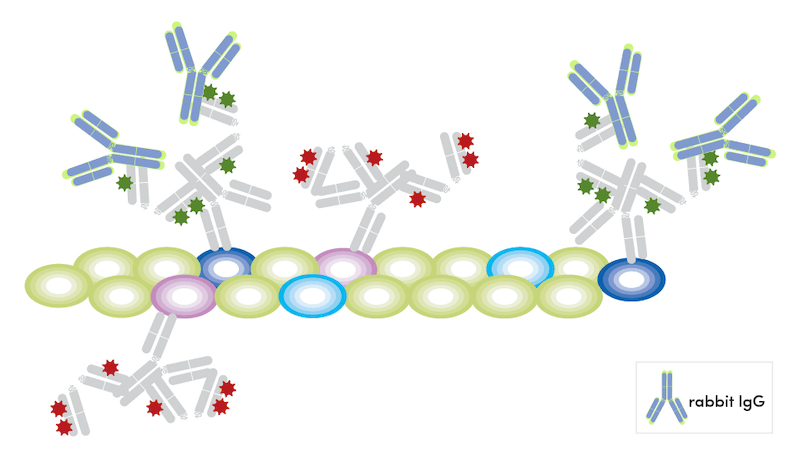
- Wash the sample three times in 1X PBS.
- Incubate the sample with fluorophore-conjugated antibodies overnight at 4° C at recommended concentrations.
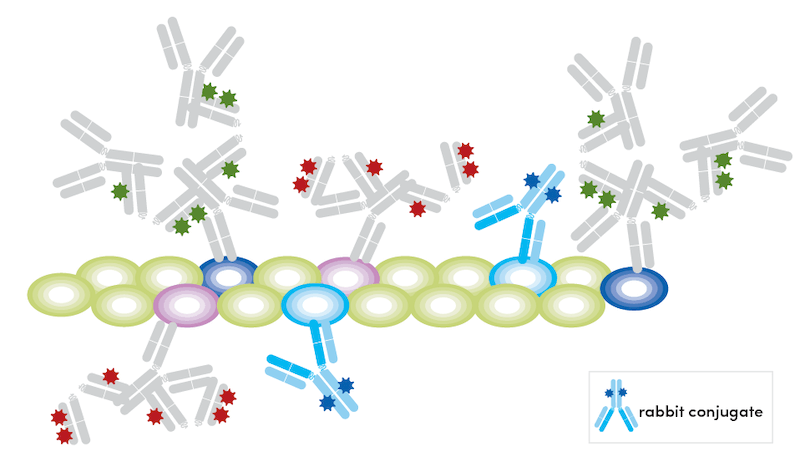
- Wash the sample three times in 1X PBS.
This technique relies on blocking free binding sites with host immunoglobulins before adding conjugated antibodies. We have successfully used this method with both rabbit (Olfm4 primary + PCNA 647 conjugate) and mouse (APP/β-Amyloid primary + GFAP 647 conjugate) antibodies, which you can see detailed below.
IF Multiplexing Using Rabbit Antibodies: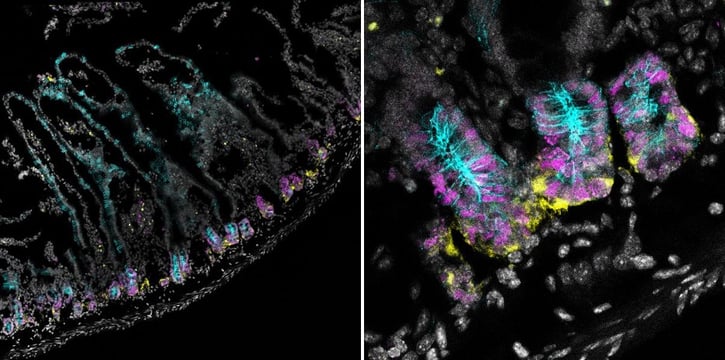
Confocal immunofluorescent analysis of mouse small intestine using Olfm4 (D6Y5A) XP® Rabbit mAb (Mouse Specific) #39141 (yellow). After blocking free secondary antibody binding sites with Rabbit (DA1E) mAb IgG XP® Isotype Control #3900, the tissue was then labeled using PCNA (D3H8P) XP® Rabbit mAb (Alexa Fluor® 647 Conjugate) #82968 (magenta) and Non-phospho (Active) β-Catenin (Ser45) (D2U8Y) XP® Rabbit mAb (Alexa Fluor® 488 Conjugate) #70034 (cyan). Nuclei are stained with DAPI #4083 (gray). Transverse small intestine was tiled at 20X (left) to demonstrate that PCNA is found in proliferating crypt cells (right).
IF Multiplexing Using Mouse Antibodies:
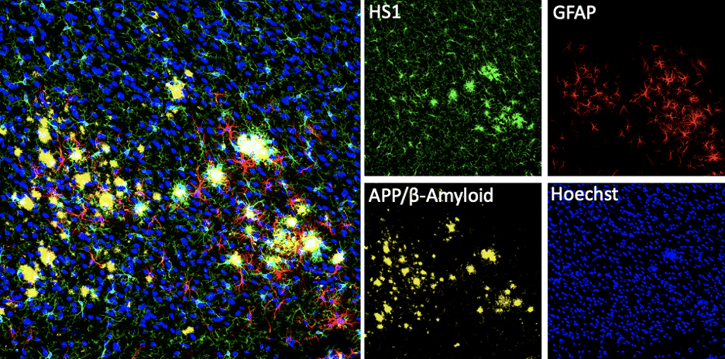
Confocal immunofluorescent analysis of mouse Tg2576 brain that overexpresses mutant human APP695. Sections were first labeled with HS1 (D5A9) XP® Rabbit mAb (Rodent Specific) #3892 (green) and APP/β-Amyloid (NAB228) Mouse mAb #2450 (yellow). After blocking free secondary binding sites with Mouse (G3A1) mAb IgG1 Isotype Control #5415, sections were incubated with GFAP (GA5) Mouse mAb (Alexa Fluor® 647 Conjugate) #3657 (red). Nuclei were labeled with Hoechst 33342 #4082 (blue).
Good luck with your experiments and happy multiplexing!
Still have questions about choosing antibodies for your multiplex IF experiment? Contact CST Technical Support for advice from our expert scientists.
21-LRG-75973